

Background: In psoriatic arthritis (PsA), treatment persistence is important for achieving optimal outcomes. The United States Food and Drug Administration (USFDA) approved guselkumab (a fully human interleukin [IL]-23p19-subunit inhibitor) for the treatment of active PsA in July 2020.
Objectives: To provide real-world evidence comparing treatment persistence while following USFDA prescribing guidelines (i.e., on-label persistence) for guselkumab (100 mg administered by subcutaneous [SC] injection at Week [W] 0, W4, then Q8W) versus SC tumor necrosis factor inhibitors (TNFi).
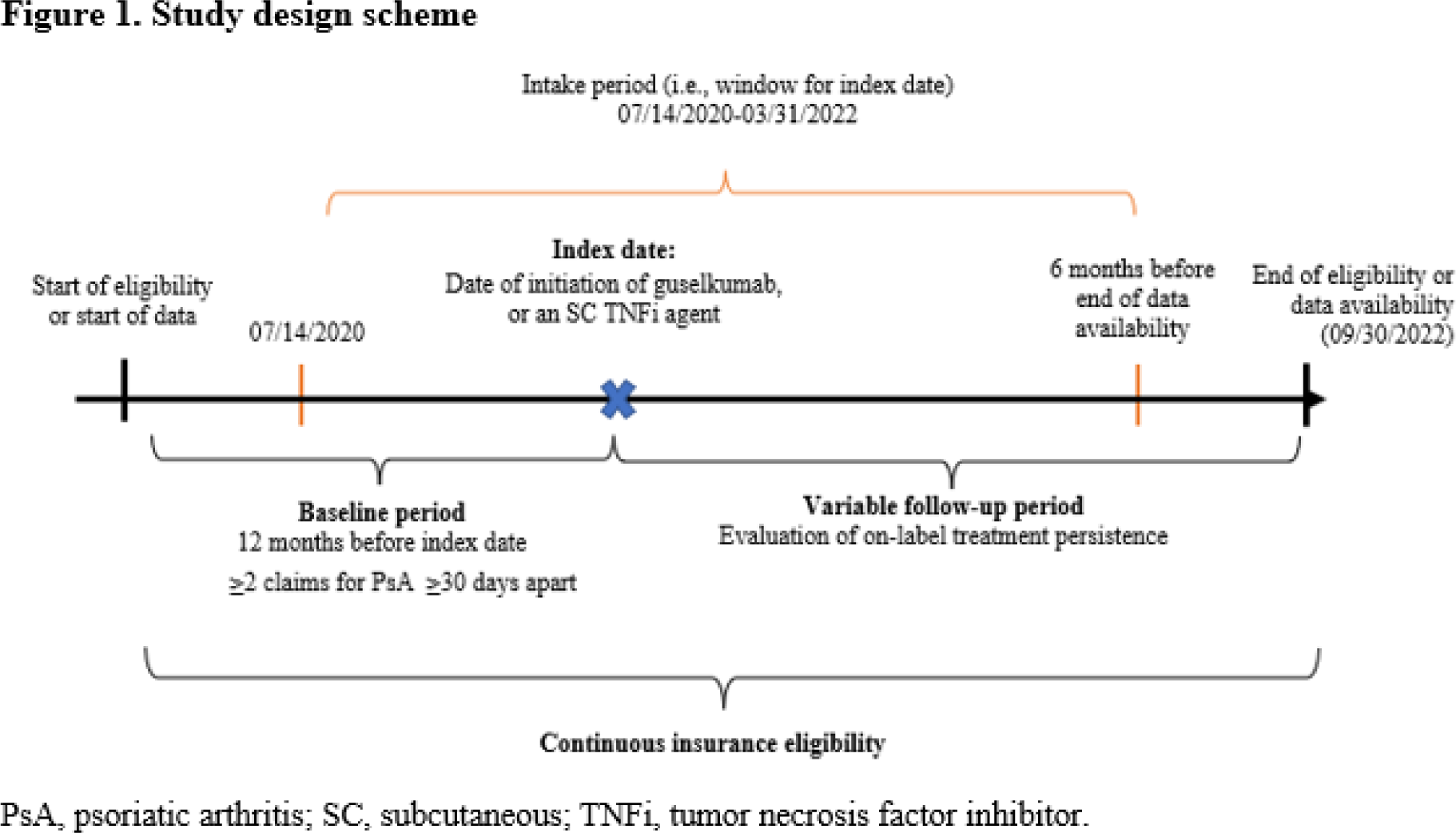
Methods: Adults with PsA newly initiated on guselkumab or the first observed SC TNFi (i.e., adalimumab, certolizumab pegol, etanercept, or SC golimumab) between 7/14/2020 and 3/31/2022 were selected from the IQVIA TM Health Plan Claims Data. The first claim for guselkumab or a SC TNFi was defined as the index date; study cohorts included bio-naïve and bio-experienced patients. Baseline characteristics were assessed during the 12-month pre-index period; the follow-up period spanned from the index date until the earliest date between the end of the continuous insurance eligibility and the end of data availability (09/30/2022; Figure 1 ). On-label persistence of the index agent was defined as the absence of treatment discontinuation or any dose escalation/reduction relative to the USFDA label dosing instructions for each respective agent. Discontinuation was defined as having a gap in treatment between consecutive days of the index agent supply of twice the duration of days of supply for a claim (i.e., 2 x 56 = 112 days for guselkumab or 2 x 28 = 56 days for SC TNFi) during follow-up. Patients with any dose change were censored on the first observed date of the dose change. Guselkumab and SC TNFi cohorts were balanced for baseline characteristics, using propensity score weighting based on the standardized mortality ratio (SMR) weighting approach. On-label persistence was assessed using weighted Kaplan-Meier (KM) curves. A weighted Cox proportional hazards model, further adjusted for baseline biologic use, was used to compare on-label persistence between cohorts.
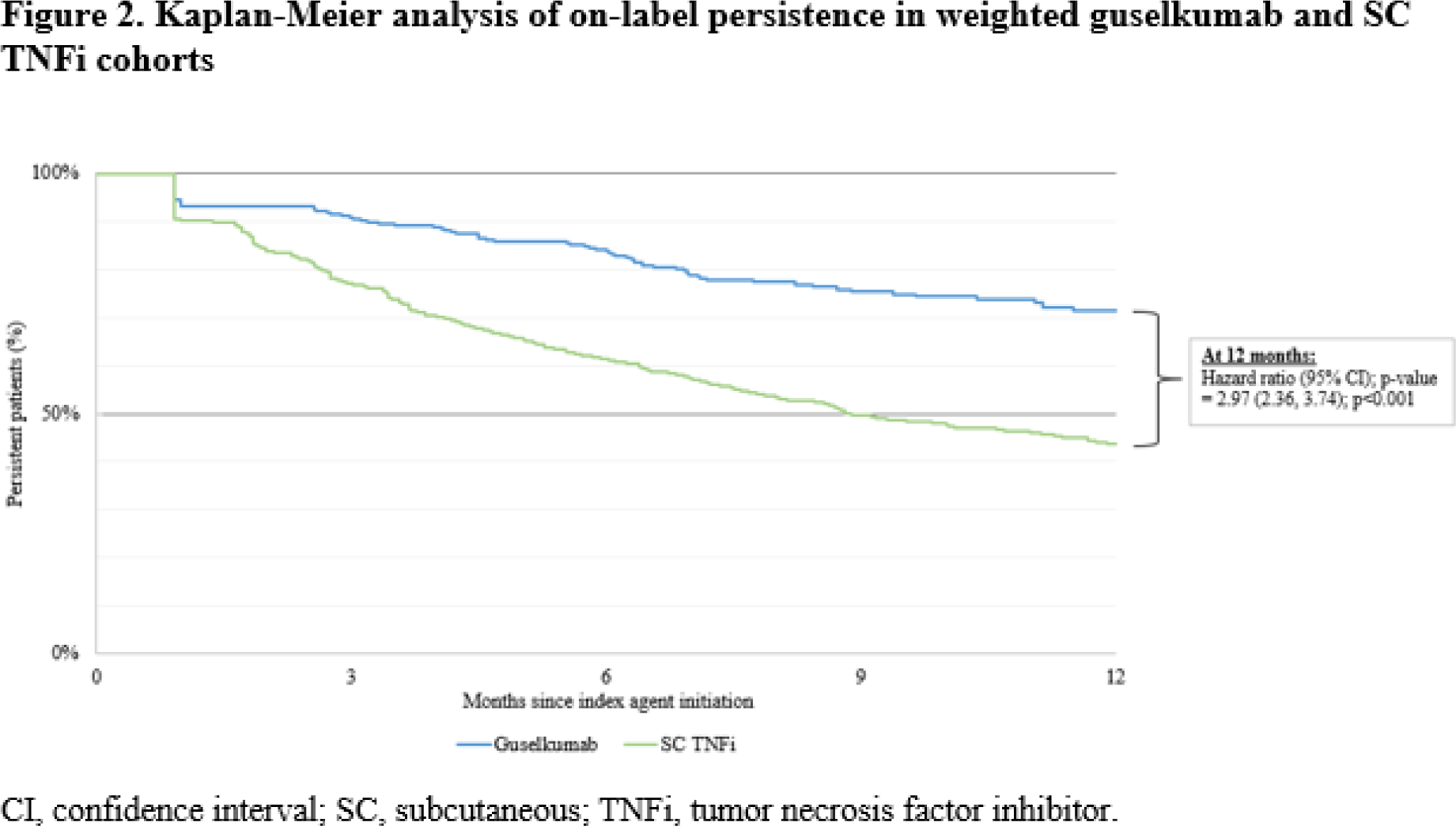
Results: The guselkumab cohort included 526 patients (mean age: 49.8 years; 61.2% female) and the SC TNFi cohort included 1,953 patients (mean age: 48.5 years; 60.2% female). After IPTW, baseline characteristics were well balanced and the mean follow-up was 12.3 months for the guselkumab cohort and 12.4 months for the SC TNFi cohort. In the guselkumab cohort, 51.5% were bio-experienced versus 16.7% for SC TNFi. Median time to discontinuation was not reached for the guselkumab cohort versus 8.9 months for the SC TNFi cohort. Weighted KM rates of on-label persistence at 3, 6, 9, and 12 months were 91.2%, 84.1%, 75.9%, and 71.5%, for the guselkumab cohort, versus 77.3%, 61.6%, 50.0%, and 43.7%, for the SC TNFi cohort, respectively (all log-rank p<0.001). At 12 months, patients in the guselkumab cohort were approximately three times more likely to remain persistent on treatment than patients in the SC TNFi cohort (hazard ratio: 2.97; 95% confidence interval: 2.36-3.74; p<0.001; Figure 2 ).
Conclusion: This real-world study assessing treatment persistence in PsA using administrative claims data demonstrated that guselkumab was associated with significantly longer on-label persistence through 12 months versus SC TNFi.
Acknowledgements: NIL.
Disclosure of Interests: Natalie J. Shiff Stockholder of Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary, Employee of Janssen Scientific Affairs, LLC, Jessica A. Walsh Consultant for AbbVie, Janssen, Eli Lilly, Novartis, and UCB, Research funding from Pfizer, Merck, AbbVie, Iris Lin Stockholder of Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary, Employee of Janssen Scientific Affairs, LLC, Ruizhi Zhao Stockholder of Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary, Employee of Janssen Scientific Affairs, LLC, Laura Morrison Employee of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, Bruno Emond Employee of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, Louise H. Yu Employee of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, Samuel Schwartzbein Employee of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, Patrick Lefebvre Employee of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, Dominic Pilon Employee of Analysis Group, Inc., a consulting company that has received research funding from Janssen Scientific Affairs, LLC, Soumya D. Chakravarty Stockholder of Johnson & Johnson, of which Janssen Scientific Affairs, LLC is a wholly owned subsidiary, Employee of Janssen Scientific Affairs, LLC, Philip J. Mease Speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB, Consultant for AbbVie, Acelyrin, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Novartis, Pfizer, Sun Pharma, UCB, and Ventyx, Research funding from AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, and UCB.