

Background: Non-medical switch is the change of treatments for non-medical reasons, generating controversy regarding bDMARD use due to the lack of evidence on its feasability. In our department, all patients treated with golimumab underwent switch to other therapies for non-medical reasons.
Objectives: To compare the clinical course, patient and physician preference, adverse events and functional limitation of patients submitted to non-medical treatment switching.
Methods: Observational, descriptive study. Patients with rheumatic diseases treated with golimumab who had the drug suspended due to a non medical indication were included. New treatment was prescribed based on rheumatologist decision. Clinical data, information about physician and patient preference, device preference, adverse events, and disability was collected at baseline, which corresponded to the first visit after suspending golimumab, at week 4 after the switch to another bDMARD or tsDMARD, and at week 12.
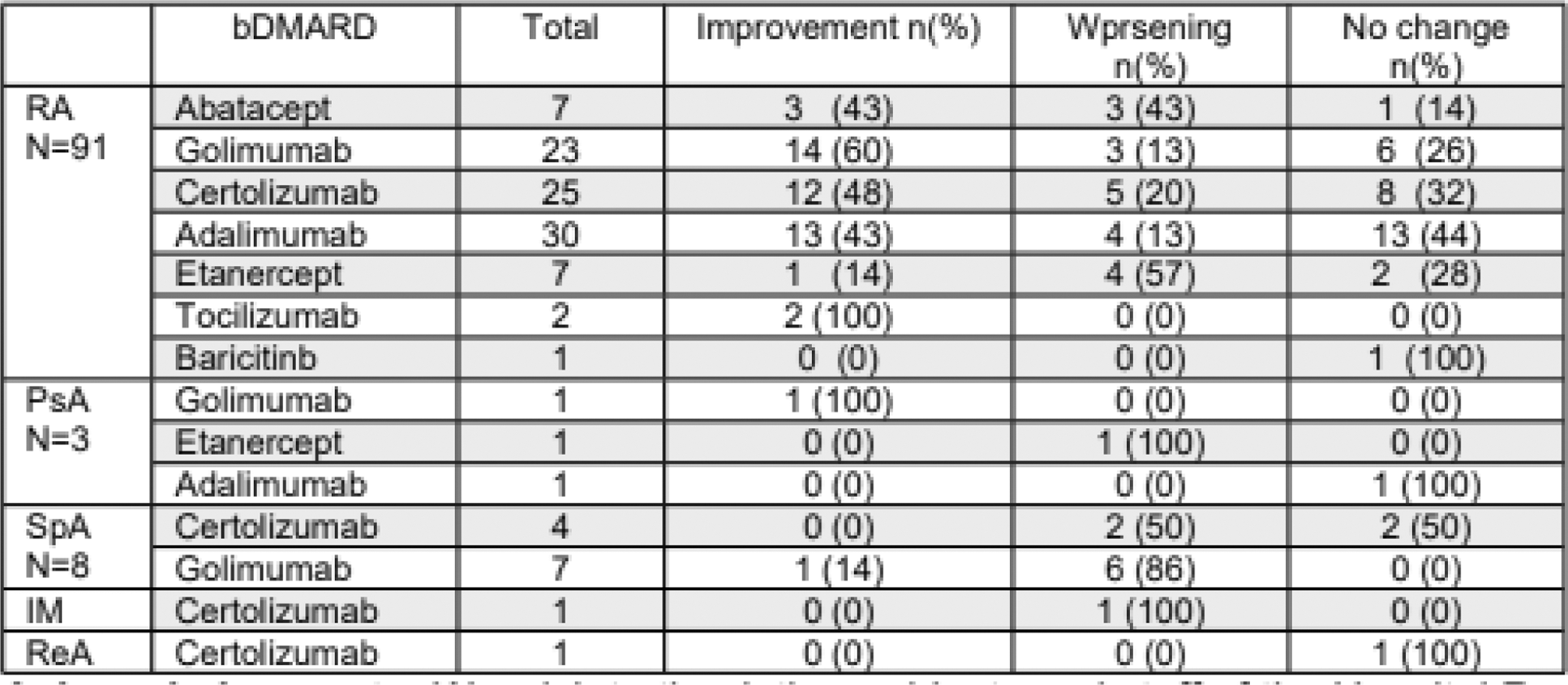
Results: 111 patients were analyzed; 99 women, mean age 57.8 years, 14.32 years since onset of their disease. Mean time with golimumab prior to suspension 30.05 months. 71 patients had history of using bDMARD or tsDMARD: 22 etanercept, 15 adalimumab, 12 infliximab, 9 certolizumab, 6 rituximab, 5 abatacept, 1 baricitinib and 1 tofacitinib. Of those, 31 thought golimumab the most effective therapy, 12 etanercept, 6 adalimumab, 5 infliximab, 2 tocilizumab, 1 rituximab, and 1 certolizumab, 4 had no preference. 41 patients at baseline used ≤10 mg prednisone. 51 patients used methotrexate, mean dose 13.08mg/w. 16 used other csDMARD. Patients spent a mean 72.93 days without bDMARD, until start of a new therapy or restart of golimumab. 84 expressed concerns at suspension, 78 due to the risk of reactivation and 45 due to the possibility of an adverse effect. According to standard of care, rheumatologists changed bDMARD: 31 to adalimumab, 31 to certolizumab, 8 to etanercept, 7 to abatacept, 2 to tocilizumab, and 1 to baricitinib. 31 patients restarted golimumab without the use of another therapy, a longer period than patients exposed to the nonmedical switch. At baseline, of the 95 patients with RA, 20 were in remission, 35 low activity, 31 moderate activity, and 9 high activity assessed by CDAI. 45 improved when starting a new therapy or restarting the previous one, 30 without changes and 20 worsened. Improvement was seen in all who started treatment with tocilizumab, 48% with certolizumab, 43% with adalimumab, and other biologics had lower response rates. All patients with baricitinib worsened as well as 57% of those with etanercept. 33 patients (34%), despite having restarted some treatment, did not improve. SpA was assessed using ASDAS/BASDAI after restarting golimumab; 6 patients remained unchanged and 1 improved. Of 4 patients with certolizumab, 2 had no changes and another 2 worsened. Regarding PsA, assessment was based on DAPSA, data is presented in Table 1. 68 preferred golimumab’s administration device, 7 a new device and 5 did not care. 36 had mild adverse events. 2 had mycobacterial infections, 1 UTI and 2 abscesses (colon and soft tissue). 11 patients had Fibromyalgia. 42 patients reported a relapse after non-medical switch. 7 required emergency care and 6 required sick leave.
Conclusion: Patients with diseases classified as stable should not be exposed to a non-medical switch. Patients with SpA were the most affected. Golimumab has the preferred application device.
Table 1. Number of patients with different rheumatic diseases showing improvement, worsening or no changes after non-medical switching of Golimumab.
REFERENCES: NIL.
Acknowledgements: We wish to thank the patients, residents and staff of the Hospital Regional 1 de Octubre, ISSSTE for their support.
Disclosure of Interests: Daniel Xavier Xibille Friedmann Astra Zeneca, Astra Zeneca, GSK, Lilly, Astra Zeneca, GSK, Lilly, Gabriel Carmona Lara: None declared, Guadalupe Olvera-Soto: None declared, Sandra Miriam Carrillo Vazquez Astra Zeneca, GSK, Janssen, Novartis, Lilly, Astra Zeneca, GSK, Janssen, Novartis, Lilly.