

Background: Rheumatoid Arthritis (RA) is a chronic multisystem inflammatory disease, in which there are different lines of treatment (conventional and biological DMARDs, among others) to control the progression of the disease. One of the most used biological treatments is Tocilizumab (TCZ), an IL-6 inhibitor. At the end of 2021, in Colombia, a supply shortage of TCZ occurred due to Covid-19 pandemic widespread use, which led to the mandatory need to change treatment with TCZ to other molecules in patients with RA.
Objectives: To evaluate the rate of therapeutic failures with the first treatment after mandatory switching from TCZ to another biologic drug or JAK inhibitor, and to know which medications were more effective in real life after the aforementioned switching.
Methods: It is a retrospective longitudinal study from September 2021 to October 2022. Descriptive statistics are presented of the types of biologicals used after TCZ switching, and which were the most effective medications in real life after the aforementioned switching. Treatment failure was defined as those patients who did not get low disease activity/remission, or in whom there was no improvement in the EULAR response measured by DAS28. An ANOVA analysis is established for the DAS28 between the groups of patients with previous use of TCZ, and those in whom there was a change in permanent biological treatment, or who returned to TCZ.
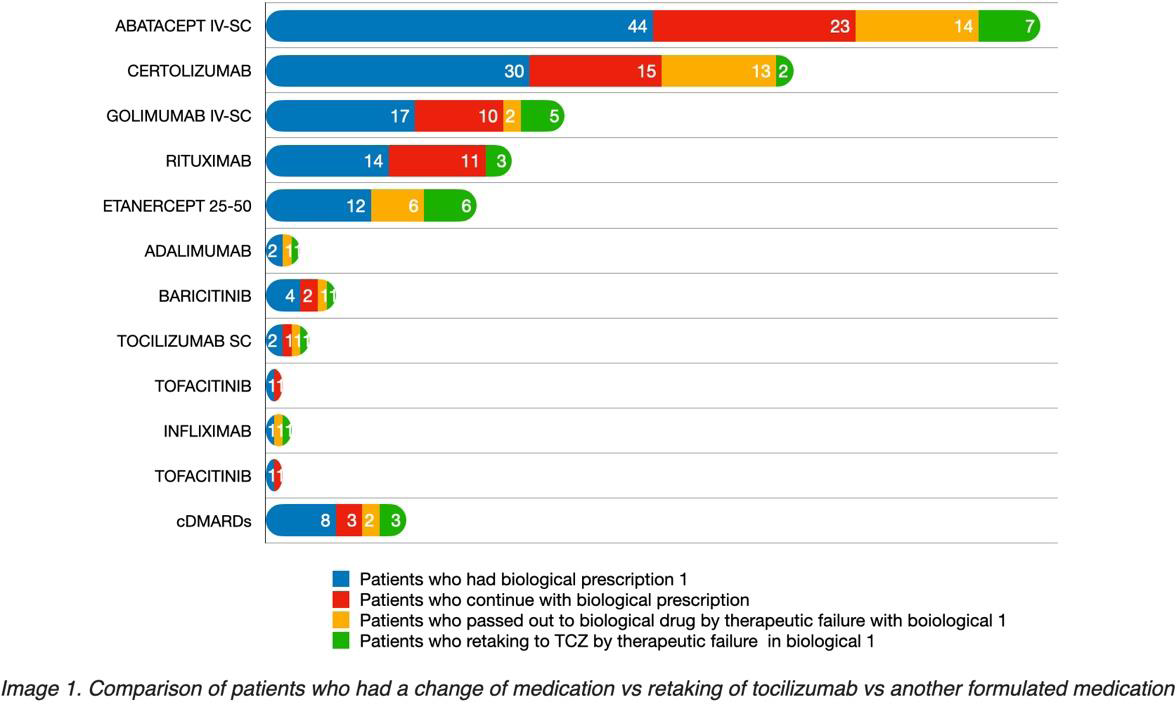
Results: 137 patients using TCZ were switched to other biological molecules or JAK inhibitors. 97% were women diagnosed with seropositive RA. The characteristics of switching to the first biological, and which were most effective are described. We describe switching to a second biological or retaking of TCZ due to failure of the first switching (Figure 1).
It was found that Rituximab with 78.5%, and Golimumab with 58.8% of effectiveness, were the most successful biologics in real life after switching post-TCZ. Abatacept and Certolizumab (52.2% and 50.0% respectively) were also relatively successful in post-TCZ switching. Between 31.8% and 43.3% of non-responding patients were transferred to a second biologic. Between 6.7% and 29.4% of non-responding patients returned to TCZ. In the case of Rituximab, 78.5% of patients continued their treatment, and only 21.5% of patients returned to TCZ. (Table 1 and Figure 1). When analyzing the DAS28, no statistically significant differences were found between the patient groups.
Conclusion: The data from this study show that the most effective molecule in real life after switching of post-TCZ is Rituximab. On the other hand, up to a third of patients return to treatment with TCZ due to failure to switch to the first biologic post-TCZ.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Pedro Santos-Moreno Abbvie, Abbott, Biopas-UCB, Bristol, Janssen, Pfizer, Roche, Sanofi, Abbvie, Abbott, Biopas-UCB, Bristol, Janssen, Pfizer, Roche, Sanofi, Nicolás Gutiérrez: None declared, Wilberto Rivero: None declared, Pedro Rodríguez-Linares: None declared, Fernando Rodriguez-Florido: None declared, Laura Villarreal: None declared, Gabriel-Santiago Rodríguez-Vargas: None declared, Adriana Rojas-Villarraga: None declared.