

Background: The etiology and pathogenesis of juvenile idiopathic arthritis associated uveitis (JIA-U) and antinuclear antibody (ANA)-positive uveitis are poorly understood. Current treatments include topical steroid therapy and methotrexate (MTX), or biologic disease-modifying antirheumatic drugs (bDMARDs). However, patients commonly fail to achieve long-lasting remission with these medications (Sen et al. 2020). It is hypothesized that the use of baricitinib, through the inhibition of JAK-STAT signalling, could target multiple cytokine pathways associated with JIA-U and ANA-positive uveitis and may provide a novel therapeutic approach to disease management.
Objectives: The aim of this study is to evaluate the efficacy and safety of baricitinib when administered to paediatric patients with active JIA-U or chronic anterior ANA-positive uveitis, who had an inadequate response to MTX or bDMARDs and are receiving topical corticosteroid eye drops at a stable dose.
Methods: This open-label, active-controlled, Phase 3 trial, was conducted at 23 centres and included children from 2 to <18 years old. Patients received oral baricitinib once daily at age-based doses (2-<9 years old: 2 mg, 9-<18 years old: 4 mg). A reference group of patients received adalimumab by subcutaneous injection every 2 weeks (20 or 40 mg based on weight). The primary efficacy endpoint was the proportion of responders at Week 24 (W24), defined according to the Standardization of Uveitis Nomenclature (SUN) criteria as a 2-step decrease in the level of inflammation (anterior chamber cells) or decrease to zero through W24 in the eye most severely affected at baseline. A Bayesian analysis was performed for the primary endpoint, based on pre-specified success criteria (the posterior probability of the treatment response rate exceeding 57% is at least 80%). Safety evaluations of baricitinib in paediatric patients with JIA-U or ANA-positive uveitis included treatment-emergent adverse events (TEAEs). TEAEs were summarised using descriptive endpoints.
Results: Among patients receiving baricitinib (N=24) the mean age was 11.6 (standard deviation [SD]: 3.5), 58.3% were female and the vast majority were White (Table 1). In the adalimumab group, the mean age was 6.6 years (SD: 2.5), 100% were female, and 80% were White (Table 1).
Table 1. Baseline Characteristics
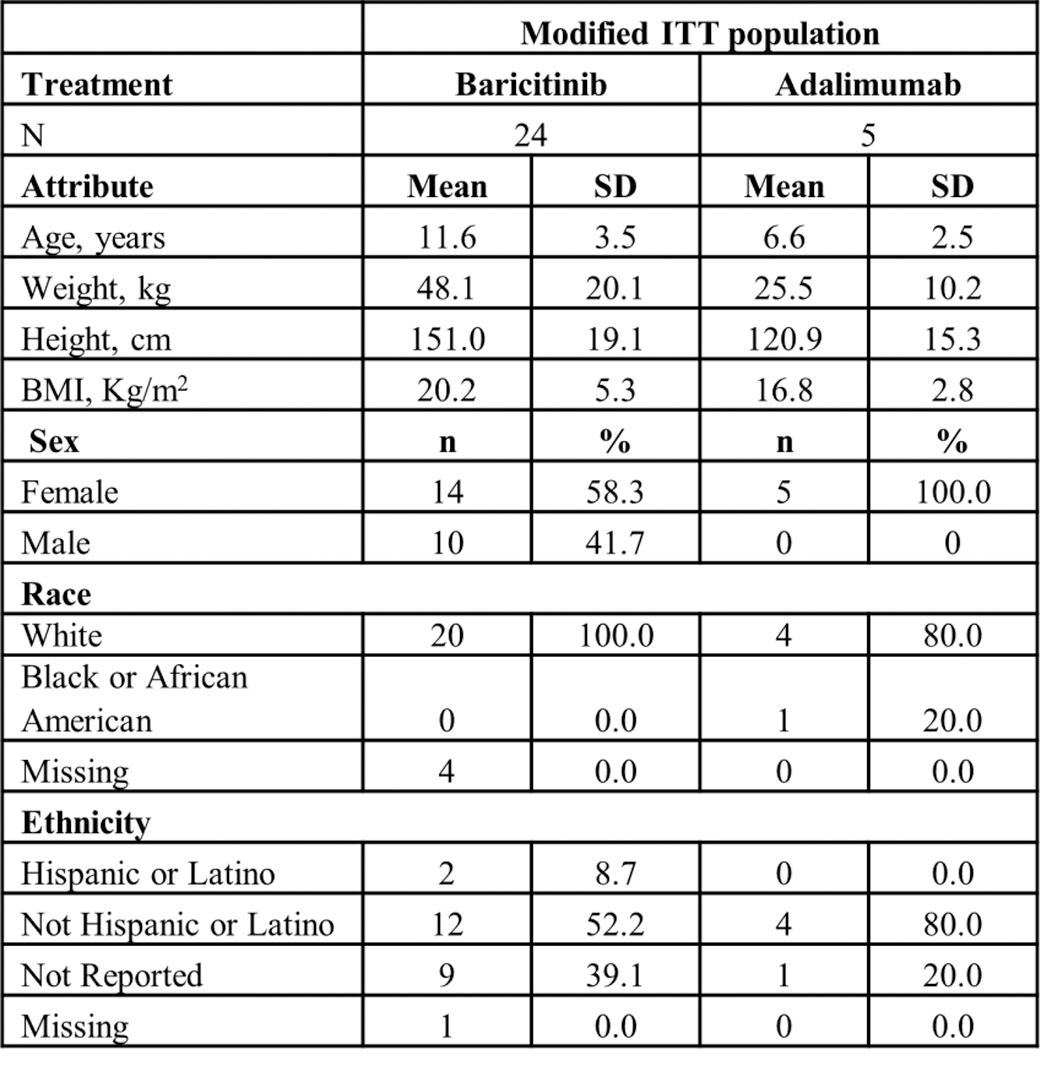
Abbreviations: BMI = body mass index; ITT = intent-to-treat; N = number of patients in population; n = number of patients in specified category; SD = standard deviation
The primary endpoint of the study was not met. In the baricitinib group, 33% of patients achieved a response at W24, resulting in 1.03% posterior probability of a response rate of >57% (Table 2). The safety data, including the observed TEAEs in baricitinib treated patients with JIA-U and ANA-positive uveitis were consistent with the established safety profile in other baricitinib indications in paediatric and adult patients. In the baricitinib group, 83.3% of patients were reported with at least 1 TEAE of which 41.7% were mild, 29.2% were moderate and 12.5% were severe.
Table 2. SUN Grade of Cells in the Anterior Chamber Response Rate at Week 24- mITT Population
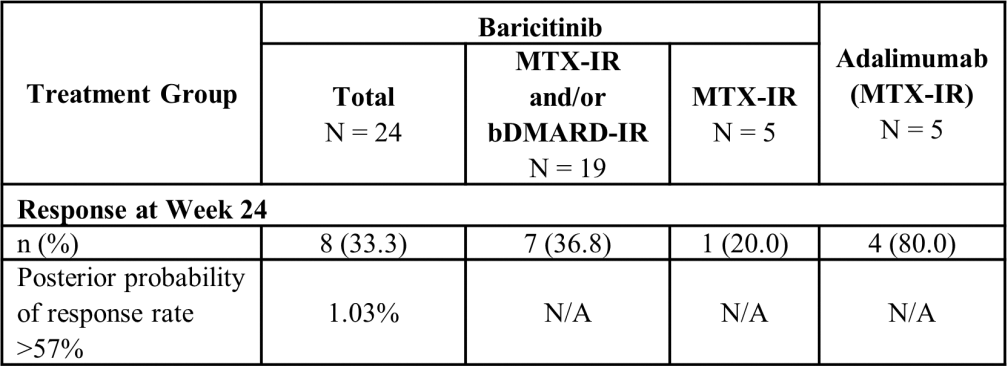
Abbreviations: bDMARD-IR = biologic disease-modifying antirheumatic drug-inadequate responder; mITT = modified intent-to-treat; MTX-IR = methotrexate-inadequate responder; N = number of patients in population; n = number of patients in specified category; N/A = not applicable; SUN = Standardization of Uveitis Nomenclature
Conclusion: The primary endpoint of the study was not met. The data provides additional information for the treatment of children with JIA uveitis refractory to both MTX and bDMARDs. Furthermore, baricitinib safety profile in this study was consistent with previous studies in children and adults with other diseases, with most TEAEs being mild or moderate.
REFERENCES: [1] Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Clinical Immunology. 2020 Feb 1;211:108322.
Acknowledgements: Caroline Murphy, Eli Lilly, for writing and editing support.
Disclosure of Interests: Athimalaipet V. Ramanan Speakers fees from Eli Lilly, Abbvie, Pfizer, Roche, Novartis and SOBI, Consulting for Eli Lilly, UCB, Abbvie, Novartis, Astra Zeneca and Alexion, Unrestricted grant from Novartis for research, Catherine Guly Speaker at JUVE Bright Investigator meeting 2019, Assisted with writing protocol for JUVE Bright study, Gabriele Simonini Un-restricted educational grant from SOBI and NOVARTIS, Stuart Keller Stockholder in Eli Lilly and Company, retirement portfolio includes mutual funds that contain a proportion of holdings in pharmaceutical stocks, Salaried employee of Eli Lilly and Company, Priyanka Sen Shareholder of Eli Lilly, Employee of Eli Lilly, Thorsten Holzkaemper Shareholder of Eli Lilly & Company, Employee of Eli Lilly & Company, Pierre Quartier Speaker for Abbvie, BMS, Chugai-Roche, Novartis, Pfizer, Sweedish Orphan Biovitrum, Consulting for Abbvie, Amgen, BMS, Chugai-Roche, Lilly, Novartis, Novimmune, Pfizer, Sanofi, Sweedish Orphan Biovitrum, Grants from Abbvie, Amgen, BMS, Chugai-Roche, Lilly, Novartis, Pfizer, Sweedish Orphan Biovitrum.