

Background: Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare inflammatory disorder, characterised by asthma, hypereosinophilia, and necrotising vasculitis of small and medium vessels. While anti-interleukin (IL)-5 treatment with mepolizumab is approved and used, oral glucocorticoids (OGCs) and other immunosuppressive agents have long been the cornerstone of treatment for EGPA but have been associated with a wide range of adverse events, and relapses are common during tapering of OGCs. Benralizumab (a monoclonal antibody against the IL-5 receptor α) may provide another treatment option that facilitates OGC-sparing, while reducing disease burden.
Objectives: To determine the effect of benralizumab and mepolizumab on OGC-sparing in the MANDARA study.
Methods: MANDARA was a Phase 3, randomised, double-blind, 52-week, non-inferiority, head-to-head study (NCT04157348) evaluating the efficacy and safety of benralizumab 1x30 mg versus mepolizumab 3x100 mg subcutaneously every 4 weeks, in adults with relapsing/refractory EGPA requiring ≥7.5 mg/day OGC (prednisolone/prednisone) for disease control ± immunosuppressive therapy. During the double-blind period, investigators were encouraged to taper OGCs for patients with no active EGPA symptoms (BVAS=0), according to standard practice and clinical judgement.
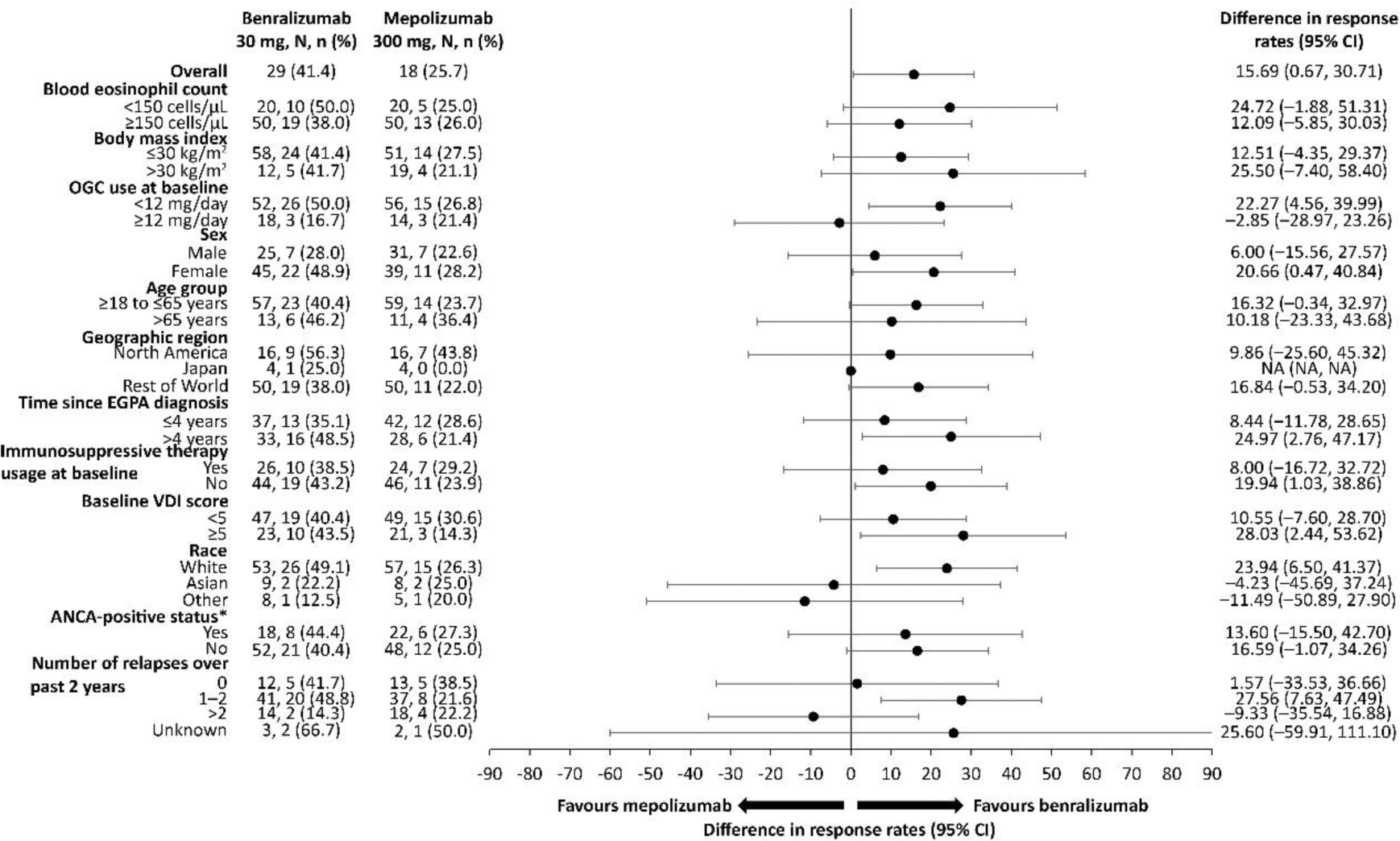
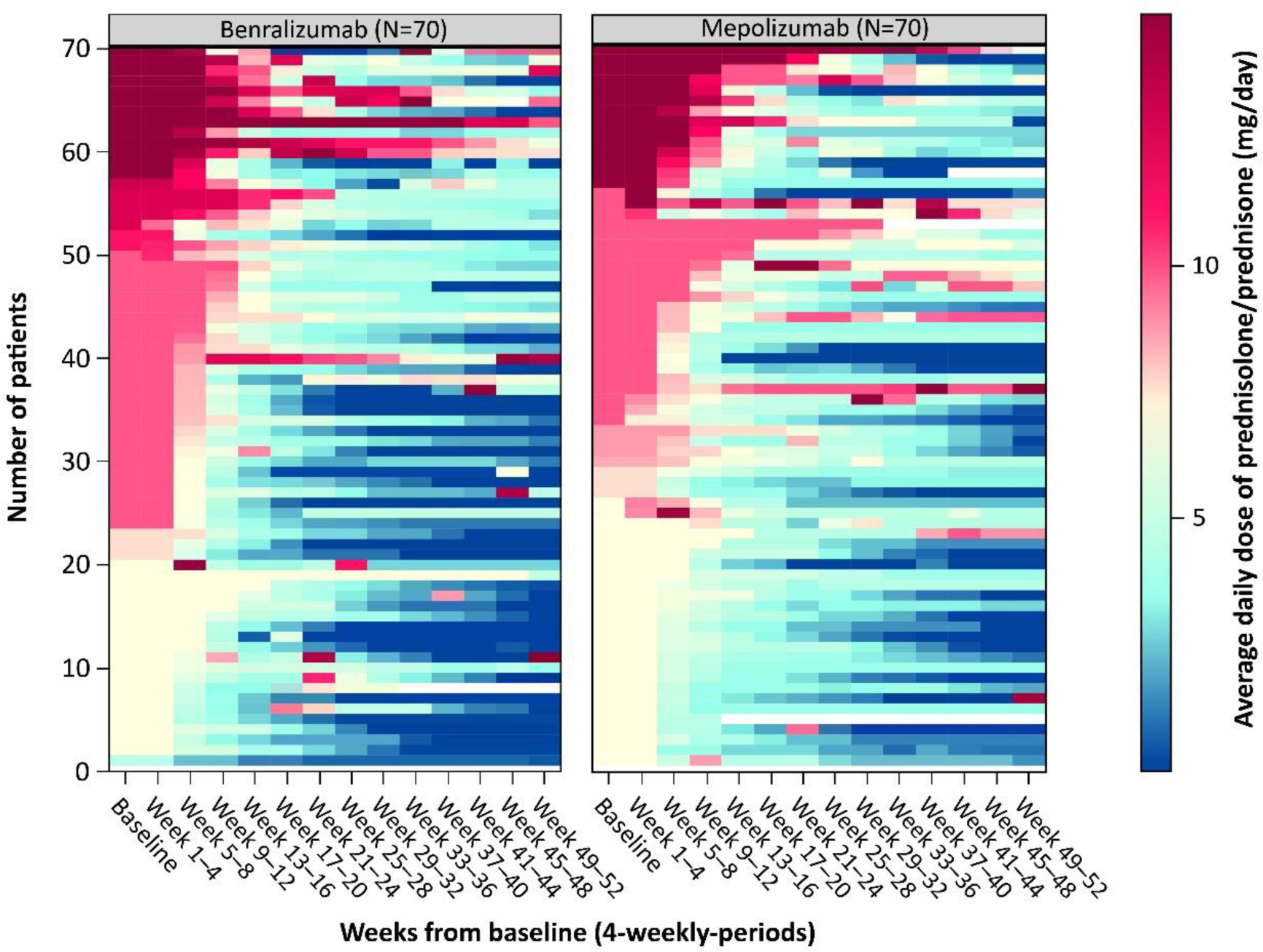
Results: 140 patients were randomised 1:1 to benralizumab (n=70) or mepolizumab (n=70); mean (standard deviation: SD) age was 52.3 (14.1) years and 60.0% were women. A subset of patients in both treatment arms achieved complete withdrawal of OGC during Weeks 48 through 52 (41.4% in the benralizumab group versus 25.8% in the mepolizumab group [difference: 15.69, 95% CI: 0.67, 30.71; p=0.0406]), and this was consistent across subgroups ( Figure 1 ). More benralizumab-treated than mepolizumab-treated patients had sustained 100% reduction in OGC use (defined as achieving 100% OGC reduction by Week 40 and maintaining reduction through to Week 52: 24.3% vs 10.0%, respectively; HR: 2.97 [95% CI: 1.26, 7.77]). Benralizumab-treated patients were able to taper off OGC regardless of antineutrophilic cytoplasmic antibody (ANCA)-status (44.4% ANCA-positive vs 40.4% ANCA-negative) and immunosuppressive therapy (38.5% with vs 43.2% without immunosuppressive use). Similar results, although with numerically lower percentages, were also observed in the mepolizumab-treated group (27.3% ANCA-positive vs 25.0% ANCA-negative and 29.2% with vs 23.9% without immunosuppressive use; Figure 1 ). A similar proportion of patients in both treatment groups achieved sustained ≥50% reduction in OGC use (defined as achieving ≥50% OGC reduction by Week 40 and maintaining reduction through to Week 52: 77.1% vs 70.0% in benralizumab vs mepolizumab groups; hazard ratio [HR]: 1.17 [95% CI: 0.79, 1.74]; p=0.3852). Benralizumab-treated patients tapered OGCs faster than mepolizumab-treated patients and maintained reduction in dose through to Week 52 as illustrated by the heatmap of OGC dose categories throughout the study period per patient ( Figure 2 ).
Conclusion: In the MANDARA study of patients with EGPA, treatment with either benralizumab or mepolizumab was associated with the ability to reduce OGC use. Compared with mepolizumab-treated patients, benralizumab-treated patients tapered OGCs faster and were more likely to fully eliminate use of OGC.
REFERENCES: NIL.
Proportion of patients achieving complete withdrawal of oral glucocorticoids during Weeks 48 through 52
*ANCA status at history or baseline.
Treatment comparison by subgroups was analysed using logistic regression using a marginal standardisation method. Covariates included treatment arm, baseline dose of prednisone, baseline BVAS, region, the subgroup and interaction between treatment arm and the subgroup (treatment arm*subgroup). ANCA, antineutrophilic cytoplasmic antibody; CI, confidence interval; EGPA, eosinophilic granulomatosis with polyangiitis; OGC, oral glucocorticoid; VDI, Vasculitis Damage Index.
Heatmap of average daily dose of oral glucocorticoids throughout the double-blind period
Values >15 mg/day were truncated to 15 mg/day.
Acknowledgements: The authors thank Claire Walton for statistical support. Caroline Ridley and Anna Mett of inScience Communications, Springer Healthcare Ltd, UK, provided medical writing support, which was funded by AstraZeneca in accordance with Good Publication Practice 2022 guidelines.
Disclosure of Interests: Bernhard Hellmich AbbVie, Amgen, AstraZeneca, BMS, Boehringer, Chugai, GSK, InflaRx, Janssen, MSD, Pfizer, Novartis, Phadia, Roche, AbbVie, Amgen, AstraZeneca, BMS, Boehringer, Chugai, GSK, InflaRx, Janssen, MSD, Pfizer, Novartis, Phadia, Roche, Michael Wechsler Amgen, AstraZeneca, Boehringer Ingelheim, Cohero Health, Equillium, Genentech, GlaxoSmithKline, Novartis, Regeneron, Sanofi–Genzyme, Sentien Biotechnologies, and Teva, Peter A Merkel Kyverna, AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chemocentryx, CSL Behring, Dynacure, EMD Serono, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, Immagene, InflaRx, Janssen, Kiniksa, Kyverna, Magenta, MiroBio, Mitsubishi, Neutrolis, Novartis, NS Pharma, Pfizer, Regeneron, Sparrow, Takeda, and Talaris, AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chemocentryx, Eicos, Electra, Forbius, Genentech/Roche, Genzyme/Sanofi, GlaxoSmithKline, InflaRx, Sanofi, and Takeda, Parameswaran Nair honoraria from Arrowhead, AstraZeneca, CSL Behring, GlaxoSmithKline and Sanofi, institution received grant support from AstraZeneca, Cyclomedica, Equillium, Foresee, Genentech, Sanofi and Teva, Arnaud Bourdin Amgen, Boehringer Ingelheim, Chiesi, Novartis, GlaxoSmithKline, Sanofi Regeneron, Amgen, Boehringer Ingelheim, Chiesi, Novartis, GlaxoSmithKline, Sanofi Regeneron, AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline, David R. W. Jayne Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Chemocentryx, Chugai, GlaxoSmithKline, Novartis, Roche, Takeda, and Vifor, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Chemocentryx, Chugai, GlaxoSmithKline, Novartis, Roche, Takeda, and Vifor, Florence Roufosse AstraZeneca, GlaxoSmithKline, Merck, and Menarini, and royalties from UpToDate, Lena Börjesson Sjö may own stock/stock options in AstraZeneca, AstraZeneca, Ying Fan may own stock/stock options in AstraZeneca, AstraZeneca, Emmanuelle Maho may own stock/stock options in AstraZeneca, AstraZeneca, Andrew Menzies-Gow may own stock/stock options in AstraZeneca, AstraZeneca, Sofia Necander may own stock/stock options in AstraZeneca, AstraZeneca, Anat Shavit may own stock/stock options in AstraZeneca, AstraZeneca.