

Background: The advent of biologic disease-modifying anti-rheumatic drugs (bDMARDs) has transformed rheumatoid arthritis (RA) management; however, their effectiveness can be hindered by infusion/injection-related reactions (IRRs).
Objectives: This study aimed to explore demographic and clinical differences between patients with RA with and without IRRs using data from the Korean College of Rheumatology Biologics (KOBIO) registry.
Methods: We analyzed data from 1,832 patients with RA participating in the KOBIO registry, grouping based on whether they experienced IRRs. Patient demographics, disease characteristics, and treatment histories were compared to identify factors associated with IRR. Switching patterns among bDMARDs over time were illustrated using a Sankey plot. Independent predictors of IRRs were identified through multivariate logistic regression analysis.
Results: Of the 1,832 RA patients, 179 (9.7%) experienced IRRs. Notable differences were observed in mean age (IRR: 49.9 years; no IRR: 54.9 years; p <0.001), C-reactive protein levels (IRR: 1.1 mg/dL; no IRR: 1.3 mg/dL; p=0.009), prior use of methotrexate (IRR: 98.3%; no IRR: 94.5%; p=0.027) and prior use of leflunomide (IRR: 60.3%; no IRR: 52.2%; p=0.039). Multivariable analysis revealed younger age as a significant risk factor for IRRs (OR 1.80, p=0.014), along with secondary Sjögren’s syndrome (OR 2.19, p=0.033) and prior leflunomide use (OR 1.49, p=0.016). Conversely, abatacept (OR 0.26, p<0.001) and tocilizumab (OR 0.42, p<0.001) were associated with a lower risk of IRRs compared to infliximab. Among TNF inhibitors, golimumab also reduced IRR risk (OR 0.34, p=0.006). After experiencing IRRs, the use of etanercept, infliximab, and adalimumab decreased, while there was an increase in the use of tocilizumab and JAK inhibitors.
Conclusion: This study highlights a significant occurrence of IRRs among patients with RA treated with bDMARDs, identifying younger age and prior use of methotrexate or leflunomide as key risk factors. These findings underscore the need for individualized patient management, suggesting that abatacept, tocilizumab or JAK inhibitors may serve as more suitable treatment alternatives for at-risk patients.
REFERENCES: [1] Salmon JH, Perotin JM, Morel J, et al. Serious infusion-related reaction after rituximab, abatacept and tocilizumab in rheumatoid arthritis: prospective registry data. Rheumatology (Oxford). 2018;57(1):134-9.
[2] Curtis JR, Chakravarty SD, Black S, et al. Incidence of Infusion Reactions and Clinical Effectiveness of Intravenous Golimumab Versus Infliximab in Patients with Rheumatoid Arthritis: The Real-World AWARE Study. Rheumatol Ther. 2021;8(4):1551-63.
[3] Gilaberte Reyzabal S, Isenberg D. Differences in the Development of Adverse Infusion Reactions to Rituximab in Patients With Systemic Lupus Erythematosus, Rheumatoid Arthritis and Non-Hodgkin’s Lymphoma-Enigma Variations. Front Med (Lausanne). 2022;9:882891.
[4] Strand V, Balsa A, Al-Saleh J, et al. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs. 2017;31(4):299-316.
Demographic and Clinical Characteristics
| Variable | Total (n= 1,832) | IRR (n= 179) | No IRR (n= 1,653) | P -value |
|---|---|---|---|---|
| Age, years | 54.4± 13.2 | 49.9 ± 13.4 | 54.9 ± 13.1 | <0.001 |
| Female | 1,508 (82.3) | 152 (84.9) | 1356 (82.0) | |
| Secondary Sjogren’s syndrome | 64 (3.5) | 10 (5.6) | 54 (3.3) | 0.108 |
| Subcutaneous rheumatoid nodule | 45 (2.5) | 8 (4.5) | 37 (2.2) | 0.075 |
| Interstitial lung disease | 109 (6.0) | 5 (2.8) | 104 (6.3) | 0.060 |
| Disease duration, years, median [IQR] | 5.2 [1.6, 11.5] | 4.7 [1.4, 10.7] | 5.3 [1.7, 11.6] | 0.408 |
| ESR, mm/hr, median [IQR] | 45 [28, 66] | 41 [26, 59] | 45 [29, 67] | 0.075 |
| CRP, mg/dL, median [IQR] | 1.3 [0.5, 2.9] | 1.1 [0.2, 2.4] | 1.3 [0.5, 2.9] | 0.009 |
| DAS28-ESR | 5.6 ± 1.1 | 5.5 ± 1.1 | 5.6 ± 1.1 | 0.521 |
| DAS28-CRP | 4.9 ± 1.1 | 4.8 ± 1.1 | 4.9 ± 1.1 | 0.317 |
| Radiographic erosion, n (%) | 713 (55.7) | 72 (54.1) | 641 (55.8) | 0.709 |
RA: rheumatoid arthritis, IRR: infusion/injection-related reaction, KOBIO: Korean Rheumatology Biologics registry, IQR: inter-quartile range, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, DAS: disease activity score
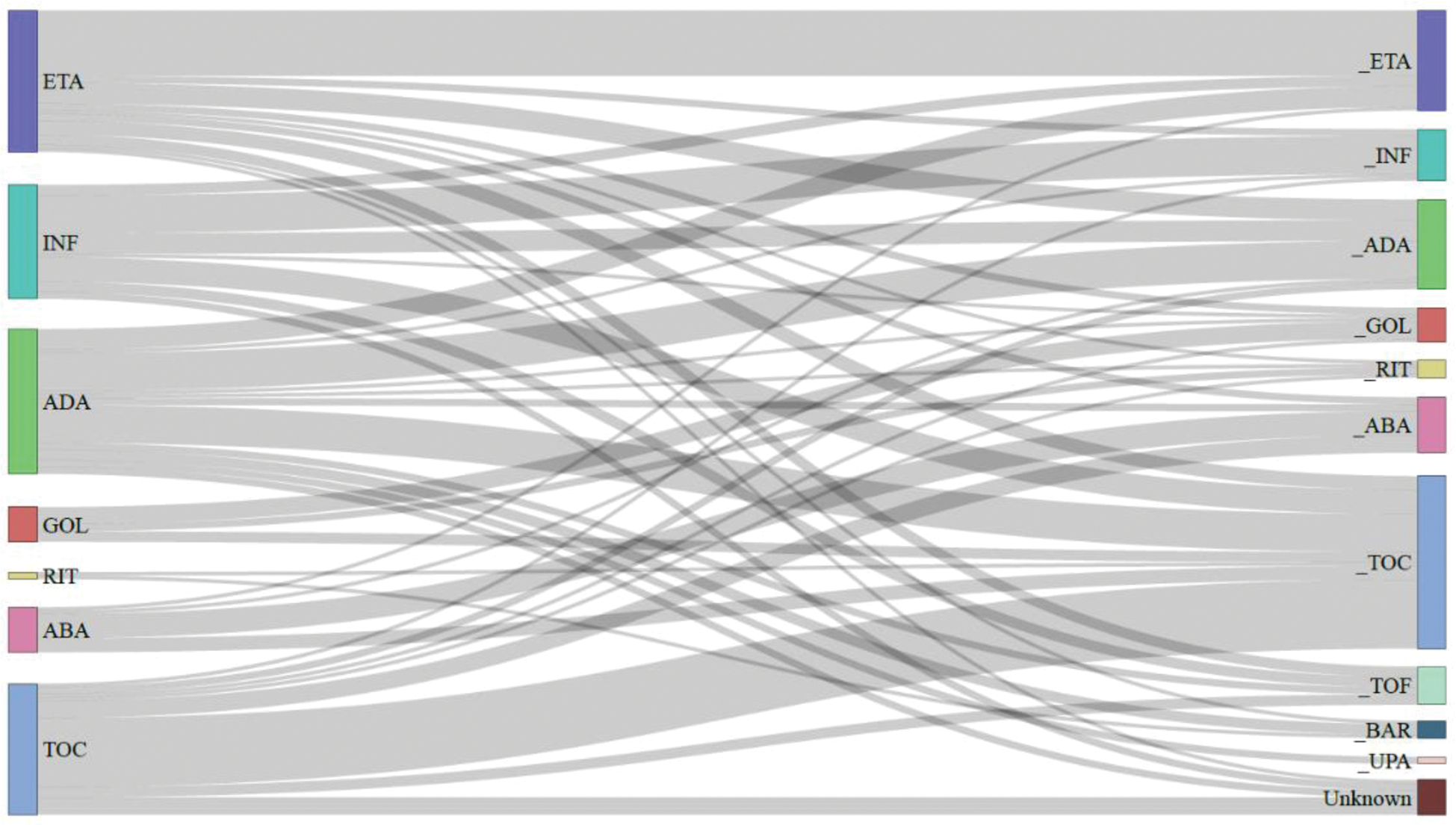
Sankey plot illustrating the biologic usage patterns at the time of infusion related-reaction occurrence
Logistic regression analysis for risk factors of IRR among patients with RA
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P -value | Adjusted OR (95% CI) | P -value | |
| Young age (vs. ≥ 65 years) | 0.47 (0.30 - 0.73) | 0.001 | 1.80 (1.15 - 2.94) | 0.014 |
| Sex (vs. male) | 1.23 (0.82 - 1.93) | 0.338 | ||
| High ESR (vs. < 66 mm/hr) | 0.80 (0.54 - 1.15) | 0.232 | ||
| High CRP (vs. < 2.8 mg/dL) | 0.76 (0.52 - 1.10) | 0.155 | 0.80 (0.54 - 1.17) | 0.260 |
| Secondary Sjogren’s syndrome | 1.75 (0.83 - 3.36) | 0.113 | 2.19 (1.01 - 4.35) | 0.033 |
| Subcutaneous rheumatoid nodule | 2.04 (0.87 - 4.24) | 0.073 | 2.15 (0.88 - 4.67) | 0.069 |
| Interstitial lung disease | 0.43 (0.15 - 0.96) | 0.068 | 0.76 (0.26 - 1.76) | 0.560 |
| Prior use of methotrexate | 3.42 (1.27 - 14.0) | 0.038 | 3.19 (1.16 - 13.2) | 0.053 |
| Prior use of sulfasalazine | 1.36 (1.00 - 1.85) | 0.053 | 1.39 (1.01 - 1.91) | 0.042 |
| Prior use of leflunomide | 1.39 (1.02 - 1.91) | 0.039 | 1.49 (1.08 - 2.07) | 0.016 |
| Concomitant corticosteroid use | 0.93 (0.62 - 1.47) | 0.756 | ||
| Biologics that induced IRR or were used at enrollment (vs. Infliximab) | ||||
| TNF inhibitors | ||||
| Etanercept | 0.75 (0.46 - 1.24) | 0.257 | 0.80 (0.48 - 1.34) | 0.389 |
| Adalimumab | 0.66 (0.41 - 1.09) | 0.101 | 0.64 (0.39 - 1.06) | 0.082 |
| Golimumab | 0.38 (0.17 - 0.76) | 0.010 | 0.34 (0.15 - 0.71) | 0.006 |
| Rituximab | 0.63 (1.00 - 2.35) | 0.549 | 0.62 (0.09 - 2.33) | 0.535 |
| Abatacept | 0.24 (0.10 - 0.47) | <0.001 | 0.26 (0.13 - 0.52) | <0.001 |
| Tocilizumab | 0.40 (0.24 - 0.65) | <0.001 | 0.42 (0.25 - 0.69) | <0.001 |
IRR: infusion-related reactions, RA: rheumatoid arthritis, OR: odds ratio, CI: confidence interval, BMI: body mass index, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, TNF: tumor necrosis factor.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (