

Background: Patients with active psoriatic arthritis (PsA) are at risk of irreversible joint damage that may significantly impact quality of life. Guselkumab (GUS), a fully human monoclonal antibody that selectively targets the IL-23p19 subunit and shows enhanced potency for inhibiting IL-23 signaling [1], has demonstrated efficacy and a favorable safety profile in patients with active PsA [2, 3].
Objectives: Report findings through Week 24 (W24) of the ongoing phase 3b, randomized, double-blind, placebo-controlled APEX study (NCT04882098), aimed at further evaluating GUS effects on clinical and radiographic outcomes in participants (pts) with active PsA.
Methods: APEX enrolled biologic-naïve adults with active PsA (≥3 tender and ≥3 swollen joints; C-reactive protein ≥0.3 mg/dL) and ≥2 joints with erosions on radiographs of hands and feet, despite previous non-biologic DMARDs, apremilast, or NSAIDs. Pts were randomized 5:7:7 to subcutaneous GUS 100mg Q4W; GUS 100mg at W0, W4, then Q8W; or PBO Q4W. The primary and major secondary endpoints were: 1) proportion of pts achieving ≥20% improvement in American College of Rheumatology response criteria (ACR20); 2) mean change from baseline in PsA-modified van der Heijde-Sharp (vdH-S) score (average of 2 readers blinded to chronological order), respectively, at W24 (both multiplicity-controlled for each GUS regimen vs PBO). Efficacy analyses include all randomized pts except those from Ukrainian sites unable to support key study operations (modified full analysis set [mFAS], N=1020); safety analyses include all pts who received ≥1 dose of study treatment through W24 (N=1054).
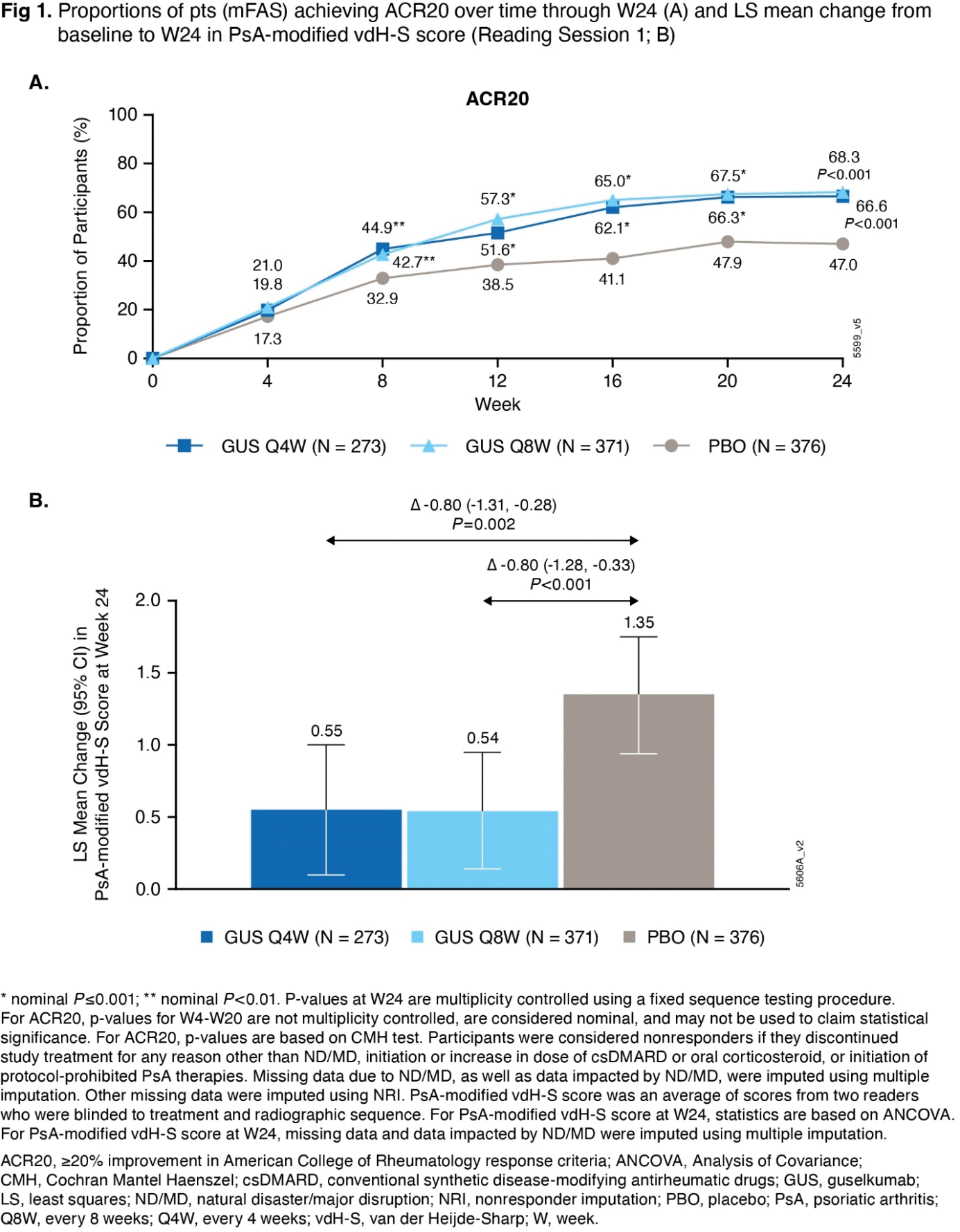
Results: In the mFAS (Q4W: 273, Q8W: 371, PBO: 376 pts), baseline characteristics were generally balanced across treatment groups. The mean age was 53 yrs; 55% of pts were male. At baseline, mean duration of PsA (7.3 yrs), PsA-modified vdH-S total (27.0) and erosion (13.5) scores, and tender (20.7) and swollen (11.9) joint counts indicated established and highly active joint disease. The primary and major secondary endpoints were met. Significantly greater proportions of GUS Q4W (67%) and Q8W (68%) vs PBO (47%) pts achieved ACR20 response at W24 ( P <0.001; Figure 1A). Pts receiving GUS Q4W and Q8W exhibited significantly less radiographic progression vs PBO at W24 (LSM changes in PsA-modified vdH-S score of 0.55 and 0.54 vs. 1.35; P =0.002 and <0.001, respectively; Figure 1B). Similar response patterns were seen for changes in joint space narrowing and erosion scores, proportions of pts with no radiographic progression, and additional clinical efficacy outcomes at W24 (Table 1). Through W24, adverse events (AEs) occurred in 38%, 42%, and 37% of pts (most commonly respiratory infections, headache, diarrhea, and psoriatic arthropathy), and serious AEs occurred in 2%, 3%, and 3% of pts, in the GUS Q4W, GUS Q8W, and PBO groups, respectively. No new safety signals were identified.
Conclusion: APEX is the first study to show significant inhibition of structural damage progression with both dosing regimens (Q4W and Q8W) of GUS, a dual-acting selective IL-23i. The GUS safety profile in these biologic-naïve pts with active PsA is consistent with that previously established for GUS across a broad range of pts with PsA, psoriasis, and/or inflammatory bowel disease [2].
REFERENCES: [1] Sachen, KL et al. Frontiers in Immunology . 2025;16:1532852.
[2] TREMFYA (guselkumab) injection, for subcutaneous use. 2024. Janssen Biotech, Inc. Horsham, PA.
[3] McInnes IB, et al. Arthritis Rheum . 2022;74:475-85.

Acknowledgements: The authors thank the patients, investigators, and trial personnel who made this trial successful. Medical writing support was provided by Erica Chevalier-Larsen, PhD, of Johnson & Johnson under the direction of the authors and in accordance with Good Publication Practice guidelines (DeTora LM, et al. Ann Intern Med. 2022;175: 1298-1304).
Disclosure of Interests: Philip J. Mease: Speaker - AbbVie, Amgen, Eli Lilly, Johnson & Johnson, Novartis, Pfizer, and UCB, Consultant - AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Immagene, Johnson & Johnson, Novartis, Pfizer, UCB, and Ventyx, Grant/research support - AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Johnson & Johnson, Novartis, and UCB, Christopher T. Ritchlin: Consultant - AbbVie, Amgen, Eli Lilly, Gilead, Johnson & Johnson, Novartis, Pfizer, and UCB, Grant/research support - AbbVie, Amgen, and UCB, Laura C. Coates: Speaker - AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Johnson & Johnson, Medac, Novartis, Pfizer, and UCB, Consultant - AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Galapagos, Johnson & Johnson, Moonlake, Novartis, Pfizer, and UCB, Grant/research support - AbbVie, Amgen, Celgene, Eli Lilly, Johnson & Johnson, Novartis, Pfizer and UCB, Alexa Kollmeier: Shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Bei Zhou: Shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Yusang Jiang: Shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Karen Bensley: Shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Koeun Im: Shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Rattandeep Batra: Shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Soumya D. Chakravarty: shareholder - stock/stock options in Johnson & Johnson, Employee - Johnson & Johnson, Proton Rahman: Consultant - AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Johnson & Johnson, Merck, Novartis, Pfizer, and UCB, Grant/research support - Johnson & Johnson and Novartis, Désirée van der Heijde: Consultant - AbbVie, Alfasigma, ArgenX, Bristol Myers Squibb, Eli Lilly, Grey-Wolf Therapeutics, Janssen, Novartis, Pfizer, Takeda, and UCB Pharma.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (