

Background: Glucocorticoids (GCs) are the cornerstone of polymyalgia rheumatica treatment. However, inability to taper GCs poses a major challenge in treatment of PMR, and long-term GC use increases risk of infection, weight gain, osteoporosis, and diabetes, which highlights the need for GC-sparing alternatives in PMR. Methotrexate (MTX) is the most commonly used GC-sparing agent in PMR and is recommended in current guidelines as the first-choice GC-sparing agent in PMR patients prone to relapses or prolonged GC use [1]. Three previous RCTs have reported conflicting results [2-4], but used fairly low doses of MTX (7.5-10 mg weekly). There remains a need for more conclusive evidence of the efficacy of MTX in PMR, especially in a 25mg/week dose.
Objectives: This study aims to evaluate the efficacy of MTX 25mg/week in achieving GC-free remission in newly diagnosed PMR patients.
Methods: We conducted a 52-week triple-blind, randomized placebo-controlled trial at the Rheumatology Department of Sint Maartenskliniek Nijmegen and Gelre Ziekenhuizen Apeldoorn and Zutphen, the Netherlands. Sixty-four patients with recently diagnosed PMR fulfilling the 2012 EULAR/ACR PMR classification criteria and treated with GC for less than 8 weeks were included, and randomly assigned 1:1 to either MTX 25 mg/week or placebo, combined with a 24-week GC-tapering protocol. Patients were stratified based on sex and level of inflammatory markers prior to inclusion, with inflammatory marker levels categorized as low or high (cut-off: ESR ≥ 70 mm/h and/or CRP ≥ 25 mg/L). Exclusion criteria included a daily GC dose > 30 mg, prior immunomodulatory use, other inflammatory rheumatic diseases, and other diseases significantly impairing assessment. Ethical approval was obtained, and all participants provided informed consent. The primary outcome was the difference in proportion of patients in GC-free remission at week 52, defined by a PMR activity score (PMR-AS) of <10 and no GC use. Secondary outcomes included relapse rates, cumulative GC dose, dose adjustments and premature discontinuation of study medication, and adverse events. Erroneous results of this study were previously presented at the ACR Convergence 2024, as a coding error resulted in some patients being analyzed in the wrong allocation group. To address this issue, the source data were audited, and all analyses were repeated.
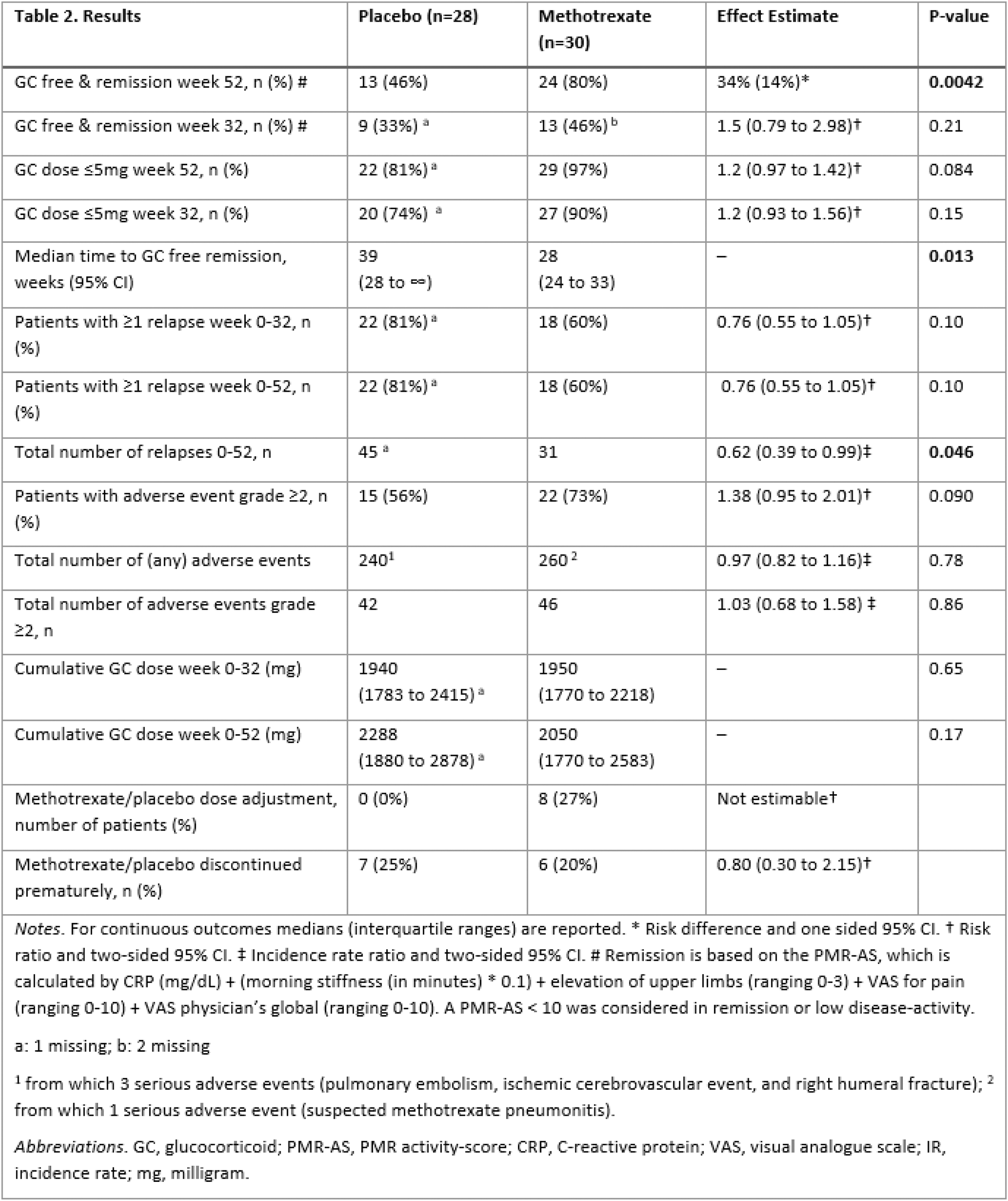
Results: From the 64 patients included, 58 were included in the final analysis. Four patients were excluded due to other diagnoses and two due to withdrawal of informed consent. Patient characteristics are detailed in Table 1. The proportion of patients in GC-free remission at 52 weeks was significantly higher in the MTX group compared to the placebo group, with a proportion of patients in GC-free remission of 80% versus 46% for methotrexate and placebo respectively (risk difference 34%, one sided 95% CI 14%, one sided p = 0.0042). Secondary outcomes showed a significantly lower incidence rate of relapse in the MTX group (incidence rate ratio 0.62, two-sided 95% CI 0.39-0.99, p = 0.046), as well as a faster time to GC-free remission (28 weeks in the MTX group (95% CI 24-33) versus 39 weeks in the placebo group (95% CI 28-∞), p = 0.013). All other secondary outcomes did not differ significantly between treatment groups, as can be seen in Table 2. Finally, there were no significant differences in total number of adverse events.
Table 1.
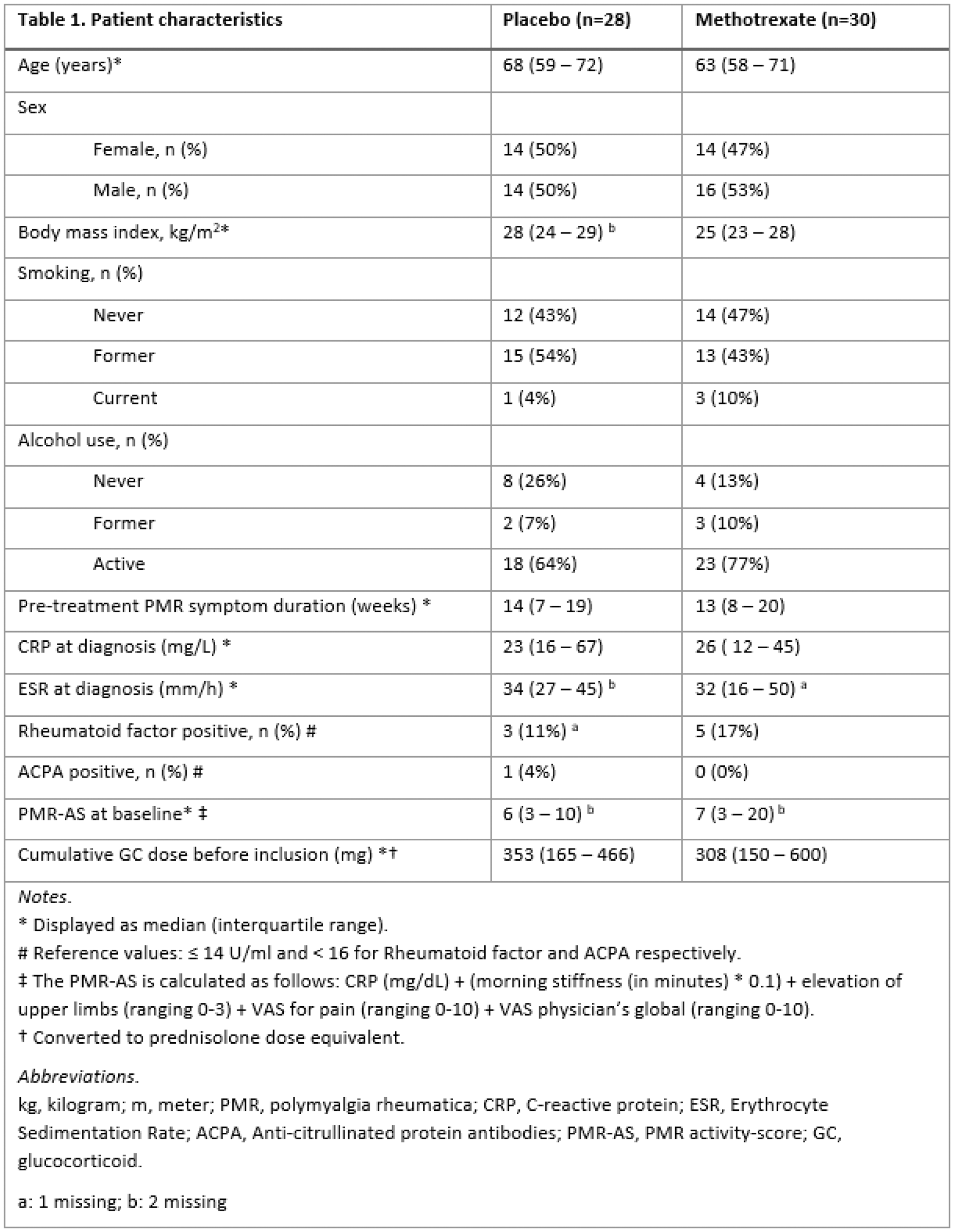
Table 2.
Conclusion: This study demonstrated a significant effect of MTX on achieving GC-free remission at 52 weeks in patients with newly diagnosed PMR. Based on these findings, MTX should be considered for newly diagnosed PMR patients, particularly those at risk of prolonged GC- therapy and associated adverse events. Additional research is needed to identify the target population at risk for prolonged GC treatment. Furthermore, research on timing of MTX initiation (at diagnosis or on flare) and comparison with other immunomodulatory agents (especially IL-6 inhibitors, JAK-inhibitors and rituximab) could be valuable.
REFERENCES: [1] Dejaco C, et al. 2015 Ann Rheum Dis. 2015;74(10):1799-807.
[2] Caporali R, et al. P Ann Intern Med. 2004;141(7):493-500.
[3] Ferraccioli G, et al. J Rheumatol. 1996;23(4):624-8.
[4] van der Veen MJ, et al. Ann Rheum Dis. 1996;55(4):218-23.
Acknowledgements: NIL.
Disclosure of Interests: Thomas E Bolhuis: None declared, Noortje Kooijman: None declared, Diane Marsman: None declared, Gijsbreght Frederik Snijders: None declared, A.A. den Broeder AbbVie, Galapagos, Pfizer, Novartis, Lilly, Sanofi, Gilead and Celltrion, Nathan den Broeder: None declared, Aatke van der Maas: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (