

Background: The goal of current therapies for systemic lupus erythematosus (SLE) is to control disease activity, reduce organ damage, and decrease long-term morbidity and mortality. Therapies providing durable clinical responses without requiring chronic immunosuppressive drugs are lacking. CD19-targeting chimeric antigen receptor (CAR) T cells have achieved durable drug-free responses in SLE patients in an academic program. Rese-cel (formerly referred to as CABA-201) is a fully human, autologous 4-1BB anti-CD19-CAR T cell therapy, designed to deeply and transiently deplete CD19 positive cells following a weight-based one-time infusion. This approach may enable an “immune system reset” with the potential for durable response without chronic immunosuppression. RESET-SLE ™ (NCT06121297) is an ongoing Phase 1/2 trial evaluating the safety and efficacy of rese-cel in 2 independent cohorts of non-renal SLE and lupus nephritis (LN).
Objectives: The primary objective is safety and tolerability within 28 days of infusion. Secondary objectives include clinical outcomes (including use of immunomodulatory agents, Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) changes, definition of remission in SLE (DORIS), lupus low disease activity state (LLDAS) status, and renal responses) and translational assessments (including CAR T cell pharmacokinetics and impact on peripheral B-cell populations).
Methods: Eligible patients are ≥18 to ≤65 years with SLE, ANA+ or anti-dsDNA+, and SLEDAI 2K ≥8 despite standard of care (SOC) therapy (non-renal SLE cohort), or active, biopsy-confirmed class III or IV ± V LN despite SOC (LN cohort). A single weight-based infusion of 1x10 6 CAR T cells/kg is administered following a preconditioning regimen (fludarabine 25 mg/m 2 /d on Days -5, -4 and -3, and cyclophosphamide 1,000 mg/m 2 on Day -3). All non-glucocorticoid immunosuppressive and antimalarial agents are stopped by Day -5.
Results: Six patients, 4 in the non-renal SLE cohort (SLE-1; SLE-2, SLE-3; SLE-4) and 2 in the LN cohort (LN-1; LN-2) have been dosed with rese-cel and have completed at least 1 month of follow-up in the RESET-SLE trial (Table 1).
Table 1.
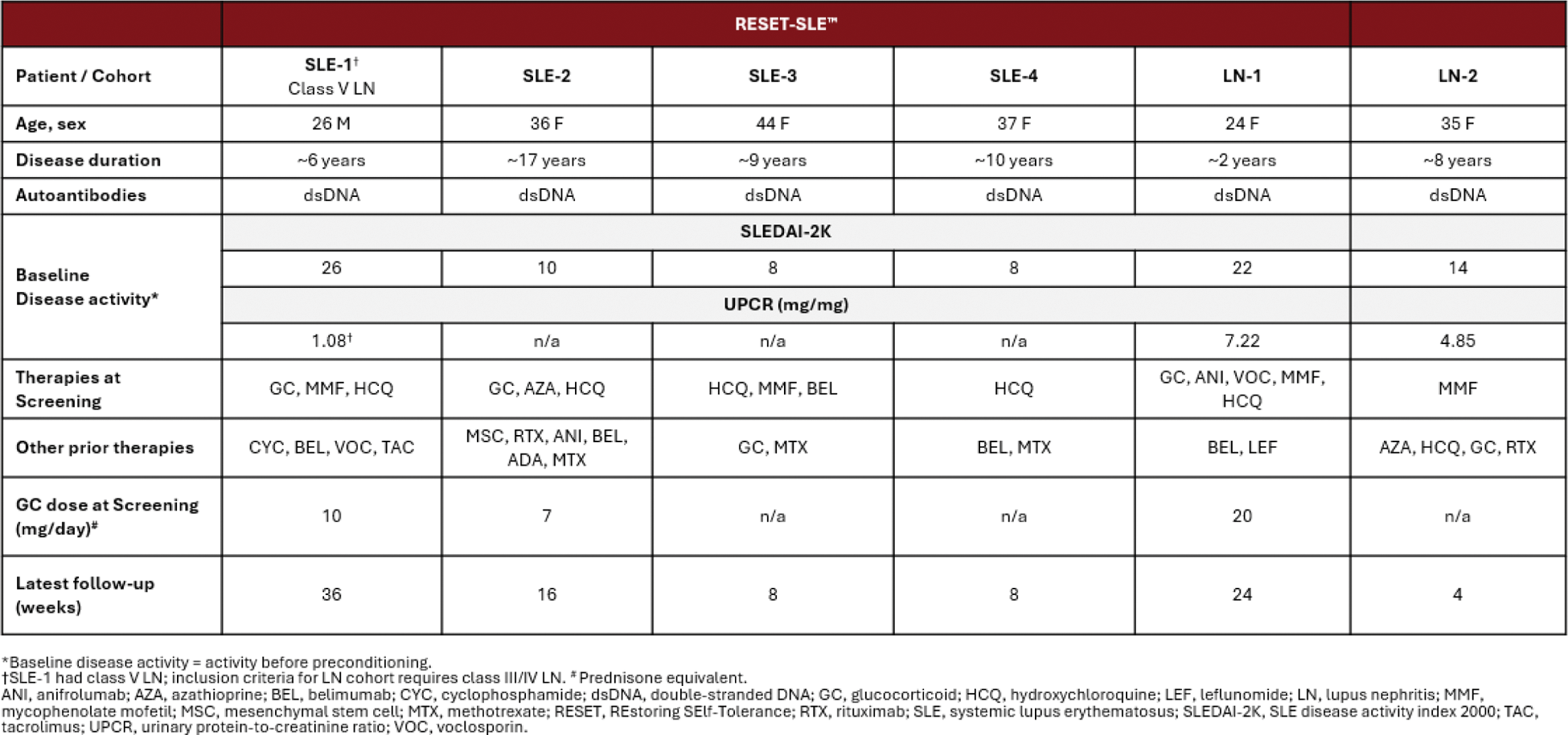
Rese-cel has been well-tolerated with Grade 1 cytokine release syndrome (CRS) (fever) reported in 2 patients, SLE-2 and LN-1, neither of whom received tocilizumab. As previously reported, the patient LN-1 experienced Grade 4 immune effector cell-associated neurotoxicity syndrome (ICANS), associated with a potential occult infection, which rapidly resolved following standard management. No other patient experienced ICANS. Clinical improvement has been observed in all 6 patients (follow up range 1 month to 9 months). Three of the 4 non-renal SLE patients achieved LLDAS by week 4, as well as DORIS remission (SLE-2 and SLE-4 by week 8, SLE-3 by week 4), and remained in LLDAS and DORIS remission as of last follow-up. The remaining non-renal SLE patient, SLE-1, with class V LN, achieved a substantial reduction in SLEDAI-2K score (26 to 8) and >50% reduction in UPCR (from 1.08 to 0.52 mg/mg) at week 28. In the LN cohort, LN-1 achieved LLDAS by week 24 and LN-2 had a reduction in SLEDAI (14 to 11) by week 4 (latest follow-up). Both LN cohort patients experienced a decrease in proteinuria. LN-1 achieved complete renal response (UPCR 7.22 to 0.45 mg/mg, steroid threshold ≤10 mg/day) by week 24 (latest follow up) and LN-2 achieved a 47% reduction in UPCR (from 4.85 to 2.56 mg/mg) by week 4. All patients remain off all SLE-related immunosuppression, and SLE-1 and SLE-2 have completed their glucocorticoid taper, while LN-1 has tapered to prednisone 6 mg/day as of the most recent follow-up visit. Translational data from 5 of the first 6 patients show that rese-cel CAR T cells peaked between days 8 and 15 post-infusion (C max ), with LN-1 also having a second peak at day 29, followed by contraction. Serum IFNγ levels peaked before or in parallel with CAR T C max . The CAR + T cells in the drug product were predominantly CD4 + for all patients and switched to a majority CD8 + at C max in 3 out of 5 patients. Peripheral B-cells were rapidly reduced within the first month post-infusion for 4 of the 5 patients. Repopulation with transitional naive B-cells occurred at week 8 for SLE-1 with insufficient follow-up for the other 4 patients. Serum levels of BAFF increased in a majority of patients post-infusion.
Conclusion: Data from SLE patients dosed with rese-cel demonstrate early immunosuppressive-free clinical remission and/or responses and favorable safety profile, including CRS events, in the setting of CAR T cell expansion and peripheral B-cell depletion. These initial data suggest the potential for rese-cel to reset the immune system in SLE, allowing patients to achieve meaningful clinical responses while off of all immunosuppressive therapies and tapering corticosteroids.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Saira Sheikh GlaxoSmithKline, AstraZeneca, Biogen, Cabaletta Bio, Aurinia Pharmaceuticals, Vimal Derebail Novartis; Travere; Bayer, Forma Therapeutics, Merck, Amgen, iCell Gene Therapeutics, Vera Therapeutics, Natalie Grover Novartis, BMS, ADC Therapeutics, Genentech, Regeneron, Gaurav Gulati: None declared, Mehrdad Abedi BMS, Gilead and Abbvie, Cytodyne, Meghan Sise Vera, Travere, Calliditas, Mallinckrodt, Novartis, Otsuka, Relay TX, Merida Biosciences, Medibeacon, Alpine Immune Sciences/Vertex, Angion, Otsuka, Gilead, Cabaletta, Novartis, EMD-Serono, Roche/Genetech, Merck, Matthew Frigault Kite, BMS, Novartis, JnJ/Legend, Cytoagents, Christopher Palma: None declared, Patrick Reagan Kite Pharma (Gilead), Caribou Bio, Genentech and Seagen (Pfizer), Cuoghi Edens: None declared, Satyajit Kosuri: None declared, Caitlin Elgarten: None declared, Jon Burnham: None declared, Jonathan Hogan Cabaletta Bio, Yvonne White Cabaletta Bio, Carl DiCasoli Cabaletta Bio, Rebecca Estremera Cabaletta Bio, Jenell Volkov Cabaletta Bio, Daniel Nunez Cabaletta Bio, Fatemeh Hadi Nezhad Cabaletta Bio, Thomas Furmanak Cabaletta Bio, Jason Stadanlick Cabaletta Bio, Larissa Ishikawa Cabaletta Bio, Zachary Vorndran Cabaletta Bio, Alexandra Ellis Cabaletta Bio, Jazmean Williams Cabaletta Bio, Steve Flanagan Cabaletta Bio, Quynh Lam Cabaletta Bio, Samik Basu Cabaletta Bio, Raj Tummala Cabaletta Bio, David Chang Cabaletta Bio.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (