

Background: Macrophage activation syndrome (MAS) is a life-threatening complication of Still’s disease (systemic juvenile idiopathic arthritis/adult-onset Still’s disease) characterized by interferon-gamma (IFNγ)-driven macrophage activation and systemic hyperinflammation. Emapalumab, an anti-IFNγ antibody, binds free and receptor-bound IFNγ, providing rapid and targeted neutralization of IFNγ.
Objectives: This analysis presents pooled efficacy and safety data from two prospective trials in patients with MAS in Still’s disease administered emapalumab.
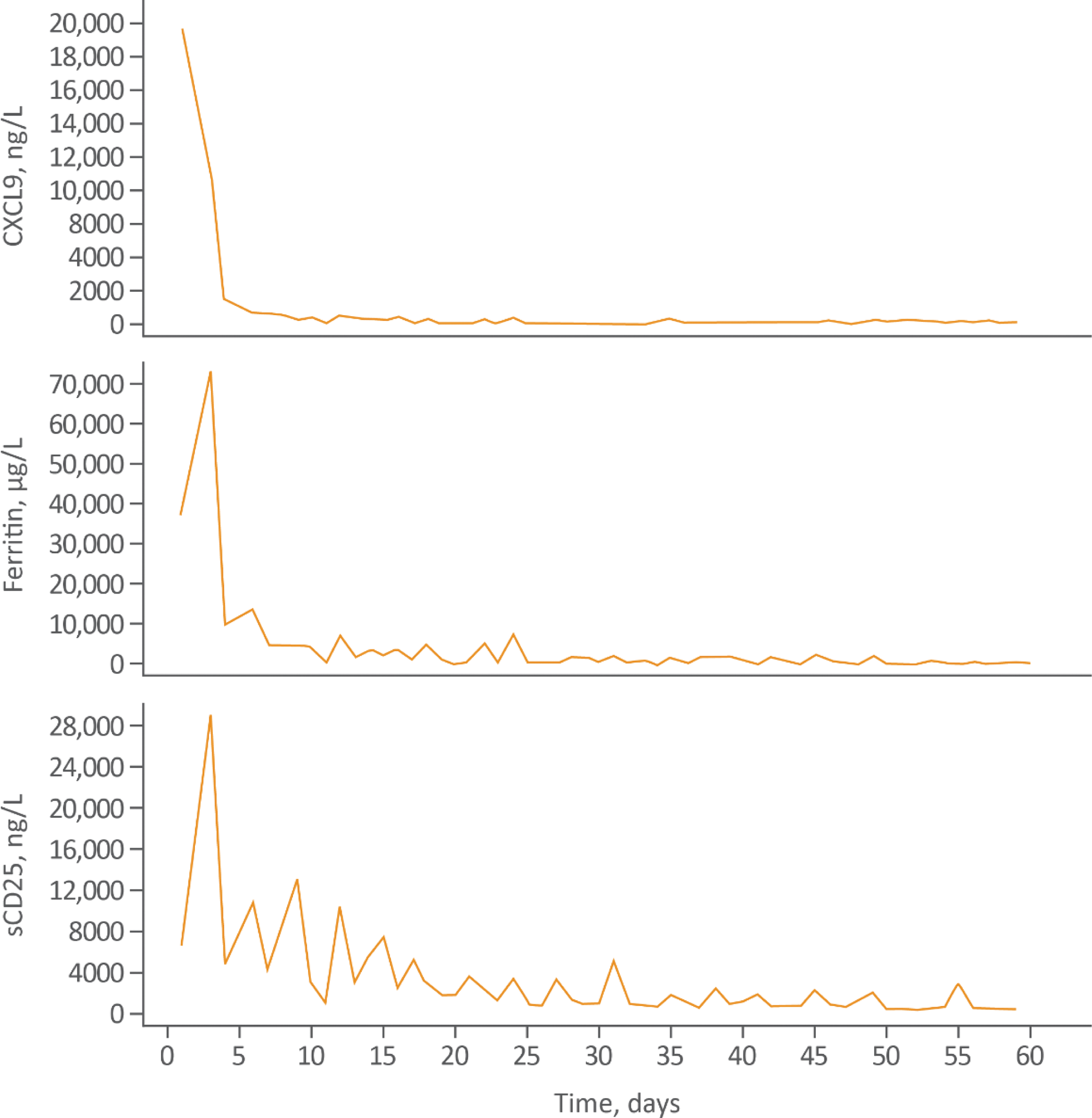
Methods: Data were pooled from two open-label, single-arm interventional studies (NI-0501-06 [NCT03311854] and NI-0501-14 [EMERALD; NCT05001737]) in patients with MAS in Still’s disease who had an inadequate response to high-dose glucocorticoids (GCs). Patients were planned to be treated with emapalumab for 4 weeks: a 6 mg/kg loading dose, followed by 3 mg/kg every 3 days from days 4–16, then 3 mg/kg twice weekly until Day 28 (or longer if insufficient clinical response). The primary efficacy endpoint was complete response (CR) at Week 8, defined as resolution of clinical signs according to investigator assessment (visual analog scale [VAS] ≤1/10) and normalization of 7 MAS-related laboratory parameters). Overall response (OR) was defined as CR or a partial response (PR). PR was defined as VAS <4/10 and normalization of ≥3 abnormal baseline MAS-relevant laboratory parameters. Other endpoints included GC tapering, survival analysis, biomarkers (chemokine C-X-C ligand 9 [CXCL9], a specific biomarker of IFNγ activity; ferritin, a marker of MAS activity; and soluble CD25 [sCD25], a marker of T-cell activity), and safety.
Results: 39 patients with an inadequate response to high-dose GCs were enrolled (31 [79.5%] females), with a median age of 12 years (range 0.9–64). Thirty (76.9%) patients had received biologics prior to study enrollment; >60% of patients had received biologics to treat the underlying Still’s disease (Table 1). 31 (79.5%) patients had been administered anakinra for MAS. At Week 8, 21 (53.8%) patients achieved a CR (95% confidence interval (CI): 37.2–69.9%) and 33 (85%) patients achieved a CR at any time. The most common CR criteria not met was lactate dehydrogenase (LDH). In a post-hoc sensitivity analysis excluding LDH, CR at Week 8 increased to 69.2% (95% CI: 52.4–83.0). 32 (82.4%) patients achieved an OR by Week 8. OR was observed as early as Day 4; median time to first OR was 2.3 weeks. 32 (82.1%) patients achieved investigator-assessed clinical MAS remission at any time (VAS ≤1 cm; first clinical remission, Day 6; median time to clinical remission, 3.3 weeks). 37 (94.9%) patients were alive at Week 8. Biomarkers of hyperinflammation rapidly reduced after initiating treatment with emapalumab (Figure 1). Clinical improvement generally paralleled the degree of IFNγ neutralization, assessed using serum chemokine CXCL9, ferritin, and sCD25. Mean (standard deviation) prednisolone-equivalent GC dose was reduced from 9.7 (9.5) mg/kg/day at Week –1 to 0.8 (0.6) mg/kg/day at Week 8. GCs were tapered to ≤1 mg/kg/day by Week 8 in 28 (72%) pts, and ≤0.5 mg/kg/day in 17 (44%) patients. No new safety concerns were identified. Six serious treatment-related adverse events were reported in 4 patients. Eight patients experienced 14 infusion-related reactions; none were serious or led to discontinuation of emapalumab infusion. Most infection adverse events were bacterial or viral and considered mild or moderate in severity.
Conclusion: Emapalumab rapidly controlled MAS in >80% of patients and enabled a reduction in GC dose in 72% of patients with MAS in Still’s disease. All patients had an initial inadequate response to high-dose GCs, and many patients also had an inadequate response to other targeted biologics prior to emapalumab initiation.
Demographics, baseline characteristics and treatment outcomes in patients with MAS in Still’s disease administered emapalumab.
| NI-0501-06
| EMERALD
| Pooled
|
|
|---|---|---|---|
| Baseline characteristics | |||
| Age, years, median (range) | 11.0 (2.0–25.0) | 13.0 (0.9–64.0) | 12.0 (0.9–64.0) |
| Sex, female, n (%) | 10 (71.4) | 21 (84.0) | 31 (79.5) |
| Biologic-experienced, n (%) | 9 (64.3) | 21 (84.0) | 30 (76.9) |
| Lung and/or hepatic involvement, a n (%) | NA | 17 (68.0) | NA |
| Receiving dialysis or hemofiltration, n (%) | |||
| Yes | 1 (14.3) | 2 (8.0) | 3 (9.4) |
| No | 6 (85.7) | 23 (92.0) | 29 (90.6) |
| Missing | 7 | 0 | 7 |
| Efficacy outcomes | n=14 | n=25 | n=39 |
| Complete response at Week 8, n (%) | 10 (71.4) | 11 (44.0) | 21 (53.8) |
| Complete response at Week 8
| 12 (85.7) | 15 (60.0) | 27 (69.2) |
| Overall response at Week 8 (complete response + partial response), n (%) | 13 (92.9) | 16 (66.7) b | 29 (76.3) b |
| Time to first overall response, weeks, median (95% CI) | 2.3 (1.4–3.0) | 2.9 (1.4–8.4) | 2.3 (1.4–5.0) |
| Clinical MAS remission at any time (VAS ≤1/10), c n (%) | 14 (100) | 19 (76.0) | 32 (82.1) |
| Time to clinical MAS remission, weeks, median (95% CI) | 3.0 (1.0–6.6) | 4.0 (2.0–5.1) | 3.3 (2.3–5.0) |
| Survival at Week 8, n (%) | 14 (100) | 23 (92) | 37 (94.9) |
Baseline data not collected in NI-0501-06.
n=24 for EMERALD and n=38 for Pooled. One fewer patient in EMERALD was considered a complete responder in the overall response analysis compared with the primary analysis, because of predefined time windows around the Week 8 timepoints (±5 days in primary efficacy endpoint analysis, and ±3 days in the overall response analysis).
As assessed at interim analysis 2, performed after 25 patients with Still’s disease in EMERALD had reached 8 weeks from the first dose of emapalumab or discontinued the study early (data cut-off date: 14 December 2023).
CI, confidence interval; LDH, lactate dehydrogenase; MAS, macrophage activation syndrome; NA, not available; VAS, visual analog scale.
Emapalumab pharmacodynamic and laboratory markers
CXCL9, chemokine C-X-C motif ligand 9; sCD25, soluble CD25.
REFERENCES: NIL.
Acknowledgements: The authors thank the patients, their families, and the investigators and their teams who took part in the study. The authors also acknowledge Stefan Duscha PhD from Sobi for publication coordination. Medical writing and editorial support, funded by Sobi was provided by Blair Hesp PhD CMPP of Kainic Medical Communications Ltd. (Dunedin, New Zealand), based on the authors’ input and direction, and in accordance with Good Publication Practice (GPP) 2022 guidelines (
Disclosure of Interests: Alexei Grom Novartis, Sobi, Kiniksa, NIH: Novartis, Sobi, SJIA Foundation, Uwe Ullman Sobi, Adnan Mahmood Sobi, Josefin Blomkvist Sobi, Brian Jamieson Sobi, Fabrizio De Benedetti Sobi, Novartis, Elixiron, Apollo, Sanofi, Abbvie, Kiniksa, Sobi, Novartis, Elixiron, Apollo, Sanofi, Abbvie, Kiniksa.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (