

Background: The 2022 update of the EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs (bDMARDs) addressed several recommendations regarding bDMARDs. Among these, recommendation 10 suggest if a bDMARD or targeted synthetic (ts) DMARD has failed, treatment with another bDMARD or a tsDMARD should be considered. However, there is currently insufficient evidence regarding the efficacy of switching to other target therapies when IL-6 inhibitors are ineffective.
Objectives: To evaluate the efficacy of switching from IL-6 inhibitors used as first-line therapy to other targeted molecular therapies in patients with rheumatoid arthritis.
Methods: We utilized a database of over 1,600 patients treated with targeted molecular therapies at Osaka Metropolitan University. Patients who initiated Phase 2 therapy with IL-6 inhibitors as their first-line bDMARD and transitioned to Phase 3 therapy were included. Retention rates at 52 weeks and changes in clinical disease activity index (CDAI) were analyzed for each targeted therapy category used in Phase 3.
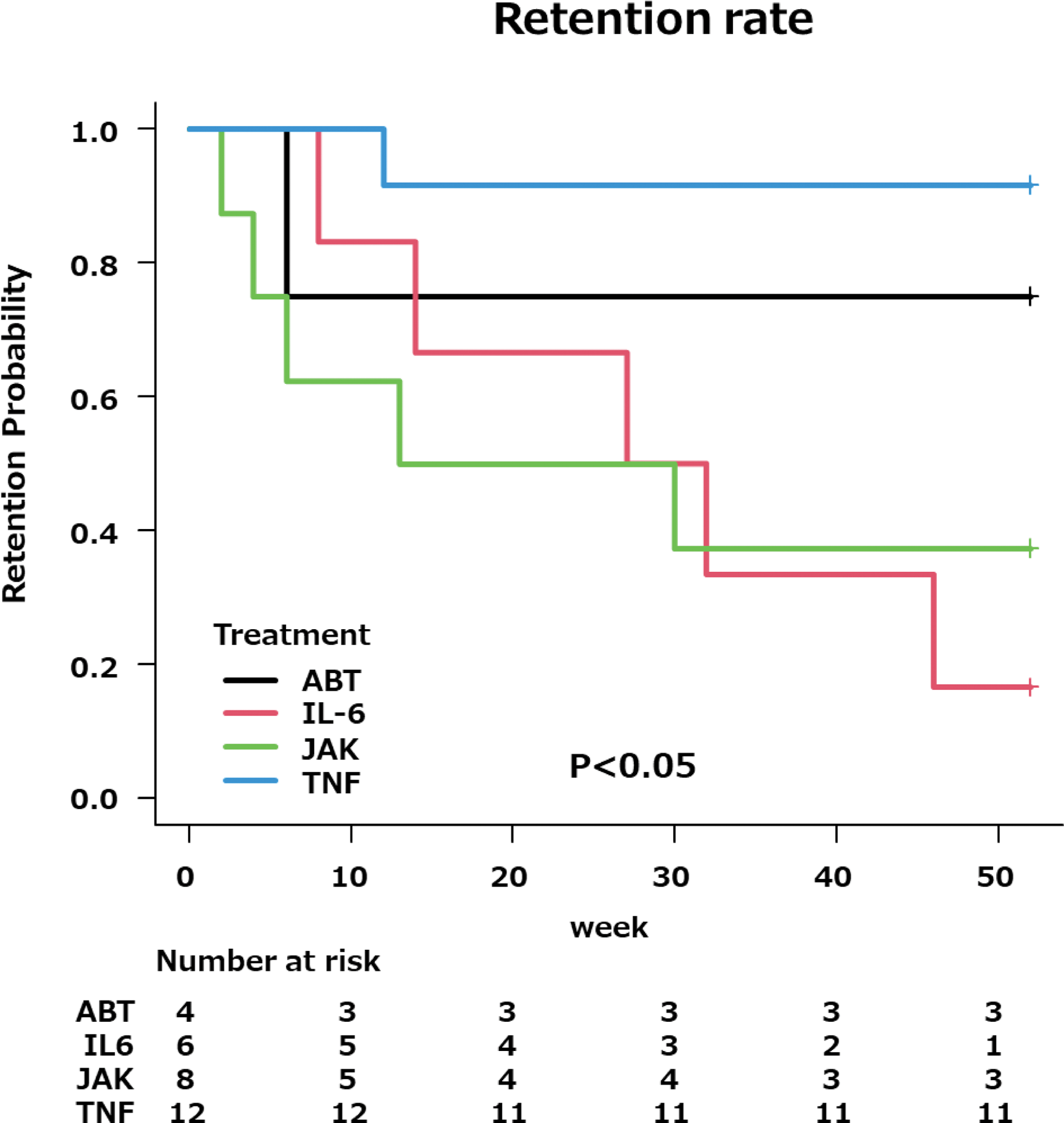
Results: The 52 weeks retention rates after switching from IL-6 inhibitors were as follows:
TNF inhibitors: 91.7% (11/12).
IL-6 inhibitors: 16.7% (1/6) (p<0.01, vs TNF inhibitors).
CTLA4-Ig: 75.0% (3/4) (p=0.383, vs TNF inhibitors).
JAK inhibitors: 37.5% (3/8) (p<0.01, vs TNF inhibitors).
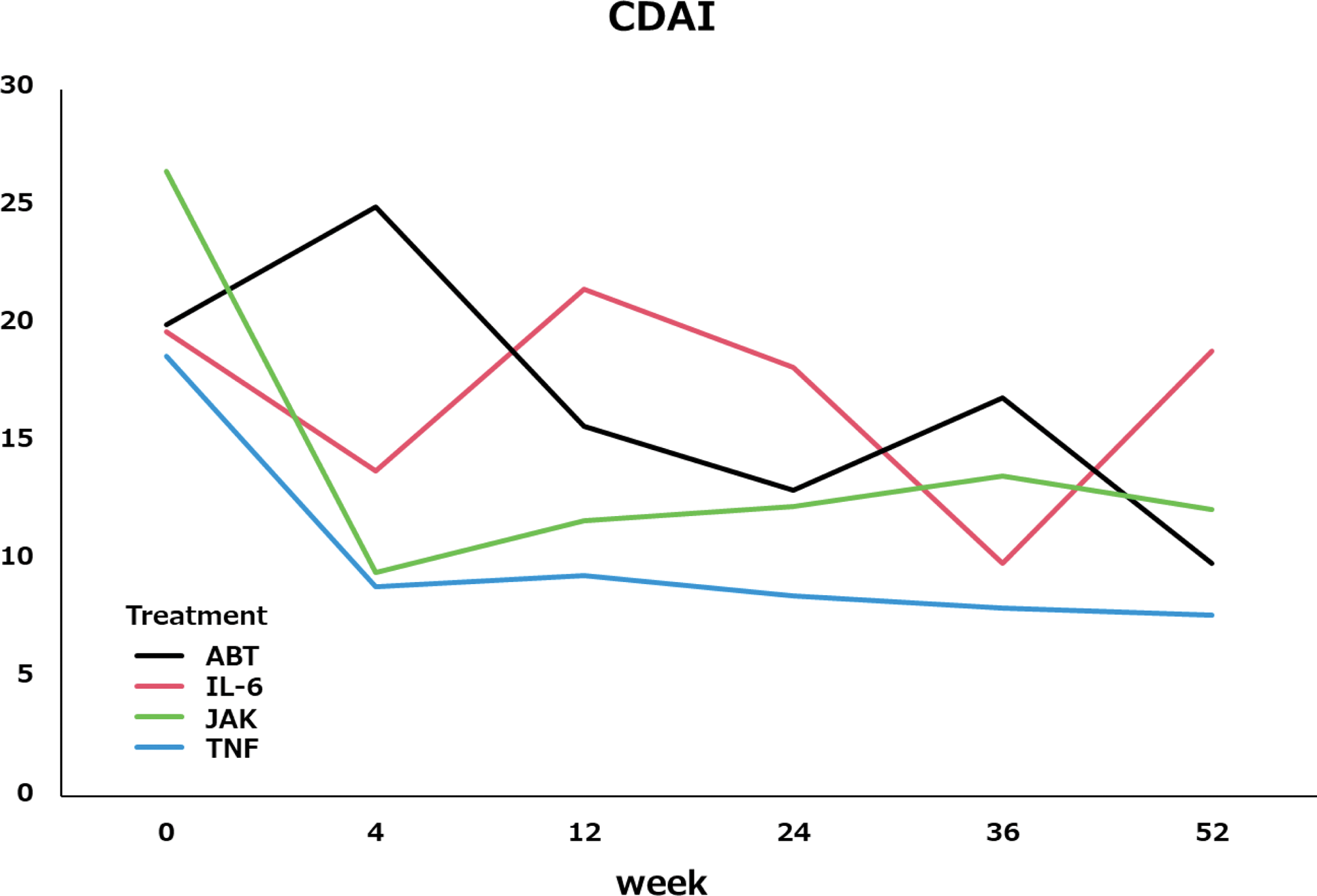
Among patients who completed 52 weeks of treatment, the mean CDAI decreased as follows:
TNF inhibitors: 18.7 to 7.7.
IL-6 inhibitors: 22.4 to 22.3.
CTLA4-Ig: 20.0 to 9.9.
JAK inhibitors: 26.5 to 12.2.
Retention rates and efficacy were higher with TNF inhibitors and CTLA4-Ig, while IL-6 inhibitors and JAK inhibitors showed lower retention rates and less favorable CDAI improvement. Switching to other IL-6 inhibitors or JAK inhibitors showed lower efficacy and retention rates, potentially due to shared pathways targeting IL-6. This suggests that switching to agents with different mechanisms of action, such as TNF inhibitors or CTLA4-Ig, may be more effective in patients with inadequate response to IL-6 inhibitors.
52 weeks retention rates after switching from IL-6 inhibitors.
mean CDAI change during 52weeks.
Conclusion: When switching from IL-6 inhibitors, consideration should be given to transitioning to targeted therapies with distinct mechanisms of action. TNF inhibitors and CTLA4-Ig demonstrated superior retention rates and efficacy, while JAK inhibitors may have limited benefit in such cases. TNF inhibitors or CTLA4-Ig should be preferentially considered in patients who fail IL-6 inhibitors.
REFERENCES: [1] Tony HP, Feist E, Aries PM, et al. Rheumatol Adv Pract, 2022;6:rkac002. Sarilumab reduces disease activity in rheumatoid arthritis patients with inadequate response to janus kinase inhibitors or tocilizumab in regular care in Germany.
Acknowledgements: NIL.
Disclosure of Interests: Tadashi Okano Abbvie, Tanabe Mitsubishi, Asahikasei, Janssen, Novartis, UCB, Eli Lilly, Astellas, Eisai, Kenji Mamoto: None declared, Yutaro Yamada: None declared, Takahito Kojima: None declared, Shohei Anno: None declared, Koji Mandai: None declared, Hiroyuki Nagata: None declared, Masahiro Tada: None declared, Kentaro Inui: None declared, Shigeyuki Wakitani: None declared, Tatsuya Koike: None declared, Hidetomi Terai: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (