

Background: Strict eligibility criteria are utilised in randomised placebo-controlled trials (RCTs) due to their ability to limit the clinical heterogeneity of the trial population and, therefore, increase the trial’s internal validity. In creating a defined homogeneous population, RCTs increase their chance of demonstrating a non-confounded clinically meaningful effect. This increased selectivity should come with a note of caution as overly stringent criteria risk a disparity between the RCT and real-world populations and poor external validity (applicability) of the RCT findings. The double-cohort design has been proposed as a method by which RCT participation can be extended, and the subsequent data’s applicability increased.
Objectives: In this study, we examine the inclusion criteria of five recent clinical trials that have shown therapeutic benefits in Sjogren’s Disease (SjD) and assess the impact of the double-cohort design on participation.
Methods: We applied the inclusion criteria of five recently published phase-two RCTs that met their primary endpoints to the UK Primary Sjogren’s Syndrome Registry (UKPSSR) to analyse the trial and registry populations. The UKPSSR is a national cohort of 1045 clinically well-characterised SjD patients who fulfil the 2002 American European Consensus Group (AECG) classification. The UKPSSR holds detailed clinical and laboratory data collected prospectively during recruitment. Inclusion criteria were extracted from ClinicalTrials.gov and published articles. The UKPSSR does not hold data on tuberculosis status or the Impact of Dry Eye on Everyday Life (IDEEL) score; therefore, these criteria were not analysed. Statistical tests and graphical rendering were performed using the R and SAS JMP Statistical Data Visualization software 23.
Results: The percentage of UKPSSR patients meeting the inclusion criteria ranged from 2.20% to 37.61% within the RCTs. The most implemented inclusion criteria were anti-Ro/SSA seropositivity and a minimum EULAR Sjogren’s Syndrome Disease Activity Index (ESSDAI) score, implemented in all five trials. 84.50% of UKPSSR were anti-Ro/SSA seropositive, whilst 40% had an ESSDAI score greater or equal to 5. A minimum EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI) was implemented in four trials, with 60.77% of the UKPSSR having an ESSPRI score greater or equal to 5. The Dazodalibep and Ianalumab trials increased their eligibility by 11.97% and 147.06%, respectively, by utilising a double-cohort design which allowed for the participation of a second cohort of “low-ESSDAI, high-ESSPRI” patients.
Eligibility of patients within UKPSSR for phase 2 randomised placebo-controlled trials . Trials utilising a double-cohort design are shown with two bars, highlighting the two cohorts eligible for trial participation. The eligibility criteria utilised by each trial and the number of UKPSSR patients fulfilling those criteria are also shown. ESSDAI, EULAR Sjogren’s Syndrome Disease Activity Index; ESSDAI (7) ESSDAI score for 7 domains (articular, cutaneous, glandular, lymphadenopathy, constitutional, biologic and hematologic); ESSDAI (8), ESSDAI score for 8 domains (constitutional, lymphadenopathy, glandular, articular, cutaneous, renal, haematological, and biological); ESSDAI (12), ESSDAI score for all 12 domains; ESSPRI, EULAR Sjogren’s Syndrome Patient Reported Index; OSF > 0.1, stimulated salivary flow greater than 0.1ml/min; OSF ≥ 0.1, stimulated salivary flow greater or equal to 0.1ml/min; Ro+, anti-Ro/SSA seropositivity.
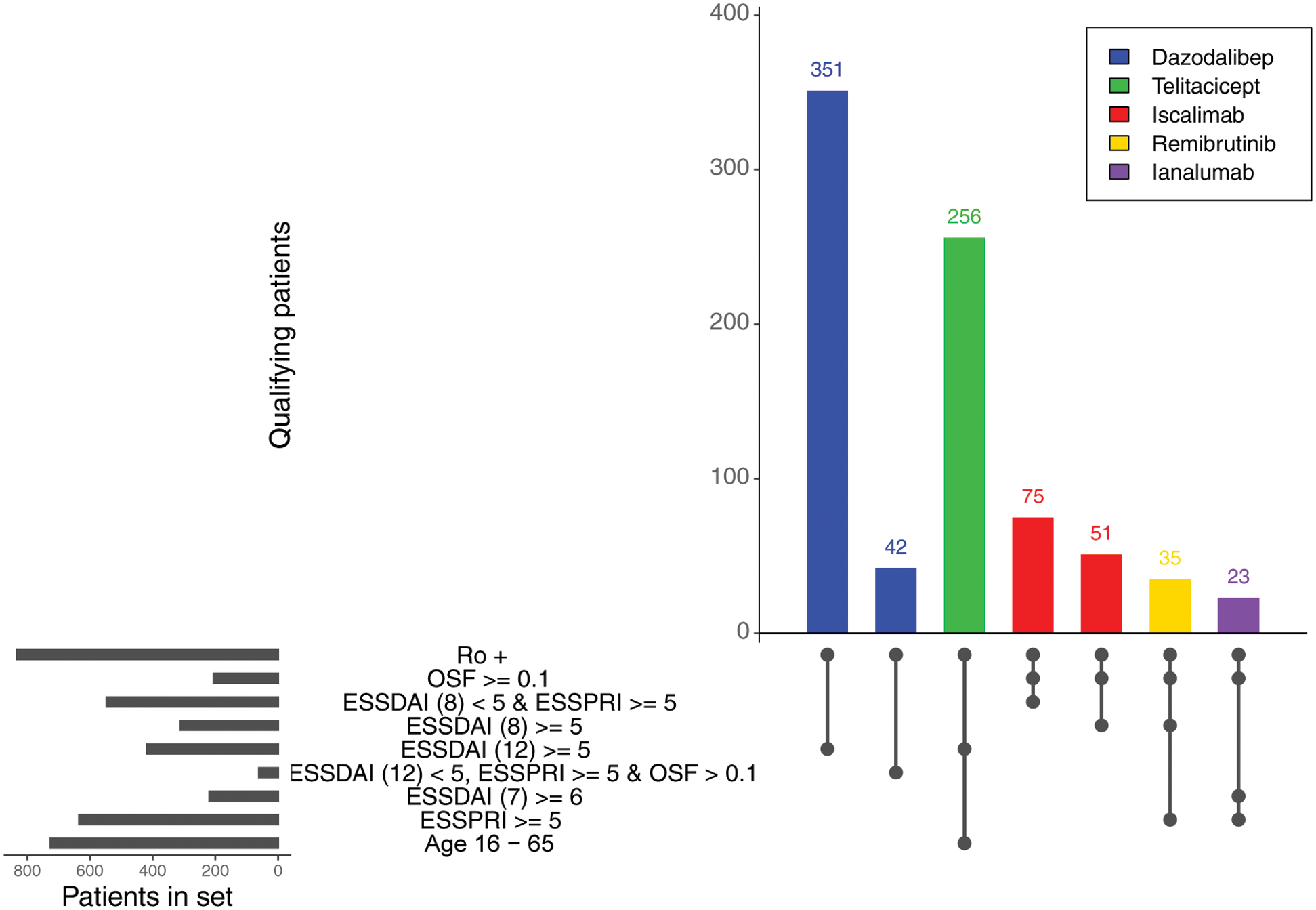
Conclusion: In this exciting era of SjD trials, an increased understanding of the benefits and limitations of trial design is vital to ensure that both clinicians and SjD patients, including those with vulnerable characteristics, have adequate treatment decision information. Our data highlight the clinical heterogeneity of the real-world SjD population, the differing impact of particular inclusion criteria, and the benefit of the double-cohort design in increasing patient inclusion.
REFERENCES: NIL.
Acknowledgements: All the patients and healthy volunteers that contribute to the UK Sjogren’s registry.
Disclosure of Interests: Joe Berry: None declared, John Casement: None declared, Kyle Thompson: None declared, Wan-Fai Ng Undertaken clinical trials and provided consultancy or expert advice in the area of Sjögren’s syndrome, Undertaken clinical trials and provided consultancy or expert advice in the area of Sjögren’s syndrome, W-FN has undertaken clinical trials and provided consultancy or expert advice in the area of Sjögren’s syndrome to the following companies: GlaxoSmithKline, MedImmune, UCB, Abbvie, Takeda, Resolves Therapeutics, Sanofi, Novartis, Bain Capitals and Argenx. No other potential conflict of interest relevant to this article was reported.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (