

Background: Immune mediated inflammatory diseases (IMID) include: rheumatoid arthritis (RA), psoriatic arthritis (PsA), axial spondyloarthritis (axSpA), Crohn’s disease (CD), ulcerative colitis (UC) and psoriasis (PsO). Although, biologic (b) and targeted synthetic (ts)DMARDs are effective, still approximately 60% of the patients are unable to reach low disease activity status. Combination therapy with two b/ts DMARDs, with a different mechanism of action, has been used in the treatment of IMID. Regarding efficacy, results are conflicting, while some safety concerns have also been raised.
Objectives: In this systematic literature review (SLR), we aimed to present the current knowledge about efficacy and safety of combination therapy in IMID.
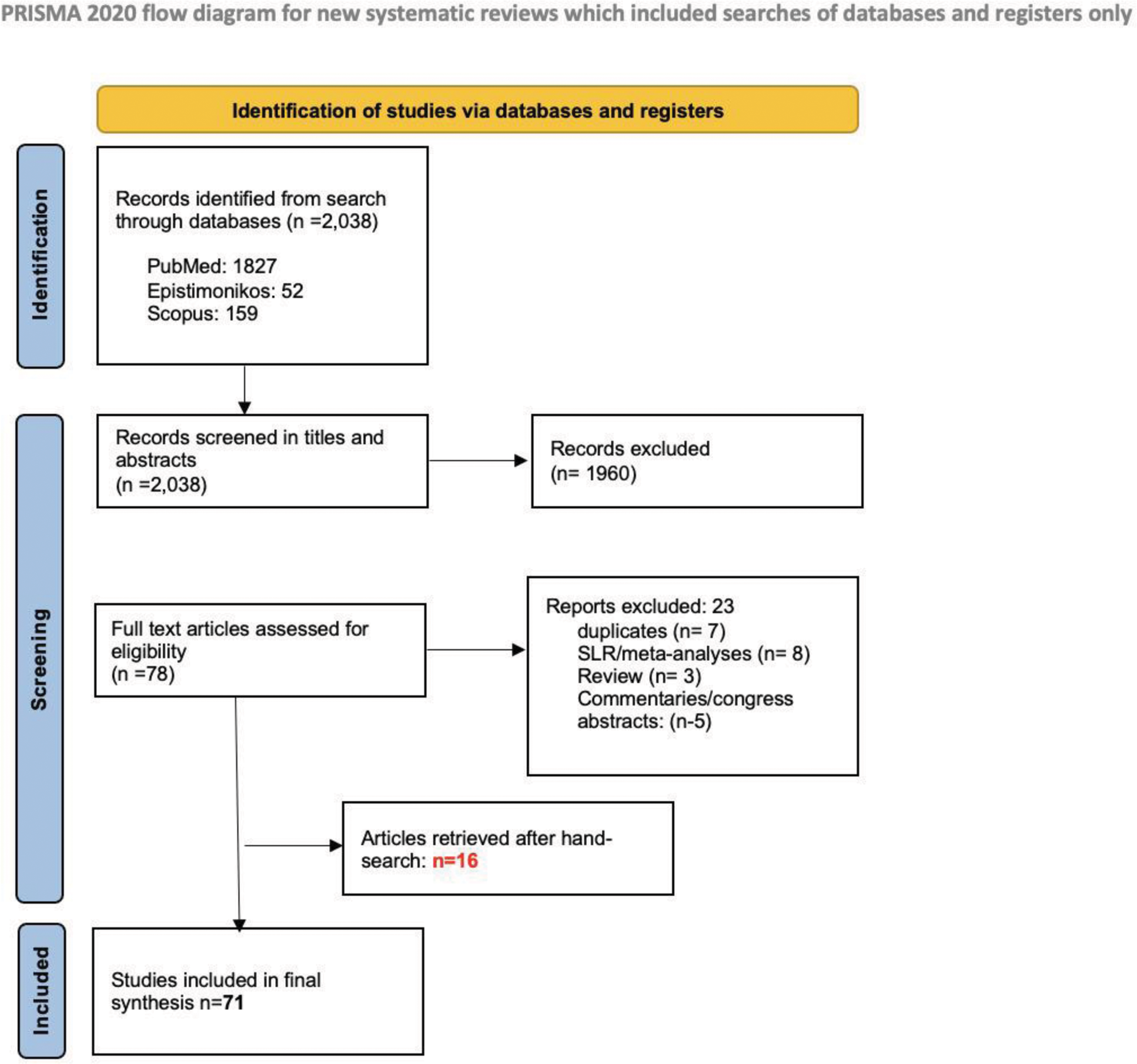
Methods: The SLR was conducted according to the patient/intervention/comparator/outcome (PICO)-structure; P: adult patients (≥18 years old) with RA, PsA, AxSpA, CD, UC or PsO. I: receive, concomitantly or alternate, two b/ts DMARDs, C: not applicable, O: efficacy (all outcomes as examined by the authors of the primary study) and safety (all adverse outcomes reported). The SLR has been registered in PROSPERO (CRD42024583183). Three databases were searched, namely: PubMed, Scopus, Epistemonikos from inception until 1/6/2024 utilising the following keywords: “inflammatory arthritis” OR “rheumatoid arthritis” OR “psoriatic arthritis” OR “axial spondyloarthritis” OR “non radiographic axial spondyloarthritis” OR “inflammatory bowel disease” OR “crohn’s disease” OR “ulcerative colitis” OR “psoriasis” AND “dual therapy” OR “bispecific therapy” OR “bimodal Therapy” OR “dual treatment” OR “bispecific treatment” OR “bimodal treatment” OR “combination biologic”. Inclusion criteria were as defined in PICO and exclusion were: non-English literature, studies related to paediatric cases (<18 years-old), editorials, reviews, congress abstracts. Case reports and case series were included. Two blinded reviewers assessed the titles and abstracts and subsequently screened the full-text articles and proceeded to the data extraction using a structured proforma sheet. In case of disagreement, a third researcher was consulted. To estimate the risk of bias, Newcastle-Ottawa (NOS) for observational studies, Revised Cochrane RoB tool for randomized trials (RoB2) for RCTs and ”Joanna Briggs Institute” critical appraisal checklist for case reports and case series were used.
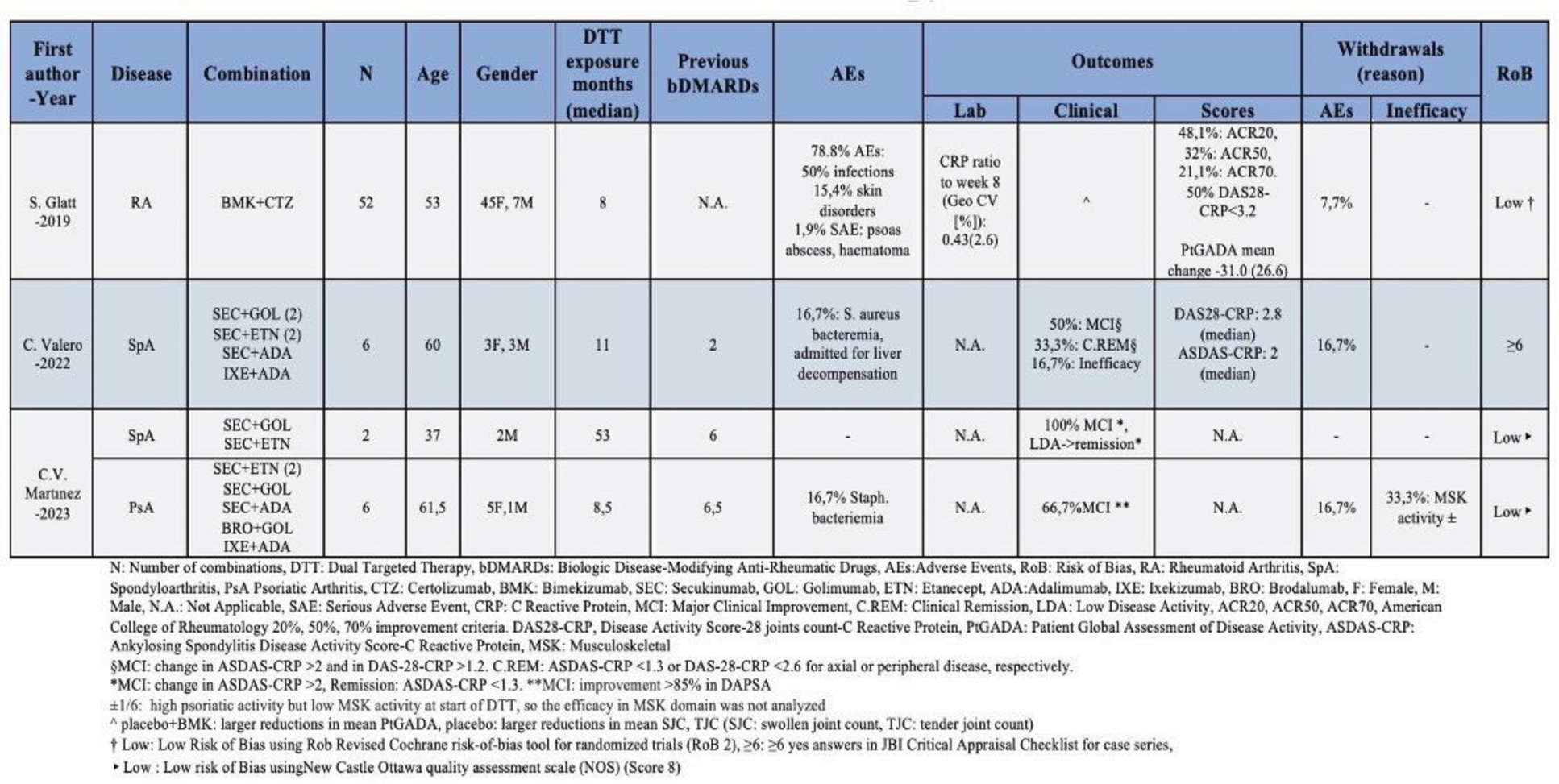
Results: Initially, 2,038 records were identified and after the elimination process (Figure 1), 71 studies were finally included (7 RCTs, 11 cohort studies, 22 case series, 31 case reports). A total of 1260 patients, previously ineffectively treated with one or more b/tsDMARDs, were included. The following categories were reported: TNFi+IL/23i (21 studies/245 patients), TNFi+IL/17i (3 studies/66 patients), JAKi+any b/tsDMARDs (9 studies/55 patients), Vedolizumab+JAKi (6 studies/14 patients), Vedolizumab+TNFi (17 studies/163 patients), Vedolizumab+IL/23i (14 studies/48 patients), Vedolizumab+ IL/17i (1 study/ 2 patients), Vedolizumab+ Ocrelizumab (1 study/1 patient)|, Apremilast+any b/tsDMARDs (14 studies/87 patients), Abatacept+any b/tsDMARDs (3 studies/289 patients), Rituximab+any b/tsDMARDs (5 studies/219 patients), Anakinra or Canakinumab+ any b/tsDMARDs (2 studies/166 patients) IL/17i+IL/23i (2 studies/4patients), TNFi+TNFi (1patient). Approximately 72.3% of observational studies scored 7 or higher in NOS, indicating a low RoB. Similarly, for RCTs, 85.7% were estimated to have a low RoB. On the JBI checklist, 72.7% of case series scored ≥8 ‘Yes’ answers, while 65% of case reports scored ≥7. Combination of TNFi with IL/23i led to favorable outcomes in about 40-60% of patients with PsA, axSpA or IBD, with safety profile being comparable to what is known from the approval studies of these drugs. TNFi and IL/17i combination therapy led to major clinical improvement in approximately 65% of patients with PsA/axSpA (understudied safety profile), whereas, based on an RCT, this strategy was safe in RA, but the additional benefit appeared to be limited (Figure 2). For strategies combining JAKi with bDMARDs, significant improvement was seen in about 50% and 60-80% of the patients with inflammatory arthritis (RA, PsA, axSpA) and IBD, respectively, without important safety issues being raised. In IBD, combination of vedolizumab with TNFi has led to improvement in 30-50% of the cases with acceptable safety profile. This figure appears to be even higher in the combination of vedolizumab with ustekinumab, although more data are needed for c. difficile infections in these patients. Data for combination of vedolizumab with JAKi are too few to interpret. Rituximab has been given in combination with TNFi with acceptable safety profile in large number of patients. Additional efficacy was not well demonstrated with 30% of the patients achieving ACR20 at week24. Apremilast has been given with bDMARDs mainly in patients with PsO leading to skin improvement without additional safety concerns. Efficacy in musculoskeletal manifestations though is unknown. Combination of bDMARDs with abatacept or anakinra, does not seem to have any benefit in RA, while is under-investigated in other diseases. Finally, few data exist for other combinations (e.g. IL/17i with IL/23i).
Conclusion: There are numerous b/tsDMARDs combinations that offer additional improvement in about half of the treatment refractory patients, across indications, without raising safety issues. With most of the studies coming from IBD and axSpA, data are more robust for TNFi/IL/23i, JAKi/bDMARD and vedolizumab/TNFi combination therapy.
REFERENCES: NIL.
Prisma flowchart
TNF- and IL/17-inhibitor combination therapy in immune mediated diseases
Acknowledgements: NIL.
Disclosure of Interests: Angeliki Zoi Lignou: None declared, Konstantinos D Vassilakis: None declared, Xenofon Baraliakos Abbvie, Alphasigma, Amgen, BMS, Cesas, Celltrion, Galapagos, Janssen, Lilly, Moonlake, Novartis, Pfizer, Roche, Sandoz, Springer, Stada, Takeda, UCB, Zuellig, Abbvie, Celltrion, Janssen, Moonlake, Novartis, Petros P. Sfikakis AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis and Pfizer, AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis and Pfizer, Jacques-Eric Gottenberg Abbvie, BMS, Chugai, Galapagos, Gilead, Lilly, MSD, Pfizer, Roche, Sanofi, UCB, George E. Fragoulis Abbvie, Novartis, Pfizer, UCB, Lilly, AEnorasis, GSK, Boheringer Ingelheim, Johnson and Johnson, Demo, Vianex.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (