

Background: Upadacitinib (UPA) has shown efficacy in rheumatoid arthritis (RA) trials, yet long-term real-world effectiveness data are scarce.
Objectives: To investigate the comparative effectiveness of UPA versus Tumour Necrosis Factor inhibitors (TNFi) in routine RA care.
Methods: Participants were included from the e-platform “Tool for Administrative Reimbursement Drug Information Sharing” (TARDIS). TARDIS collects data from all Belgian RA patients on advanced therapy during the submission of every reimbursement request for bio or ts DMARD drug initiation or prolongation. Patients starting on UPA or on a TNFi between 01/11/2020-31/12/2021, ≥18 years and DAS28>3.7 and with follow-up data were included. Effectiveness was analysed after 27 months for UPA patients and 30 months for TNFi based on the proportion of patients achieving remission (DAS-28 <2.6), low disease activity (LDA, DAS-28 ≤3.2), HAQ-DI≤0.25 (HAQ25), HAQ-DI≤0.50 (HAQ50) as well as a clinical meaningful 0.22 HAQ-DI-decrease (MCID_HAQ). Logistic regression models were constructed with these outcomes as dependant variable, and UPA vs TNFi as independent variable. To control for potential confounding, logistic models were adjusted for propensity score-based inverse probability weighting. The propensity model included age, disease duration, treatment line, and baseline joint counts, DAS28, HAQ, and PGA.
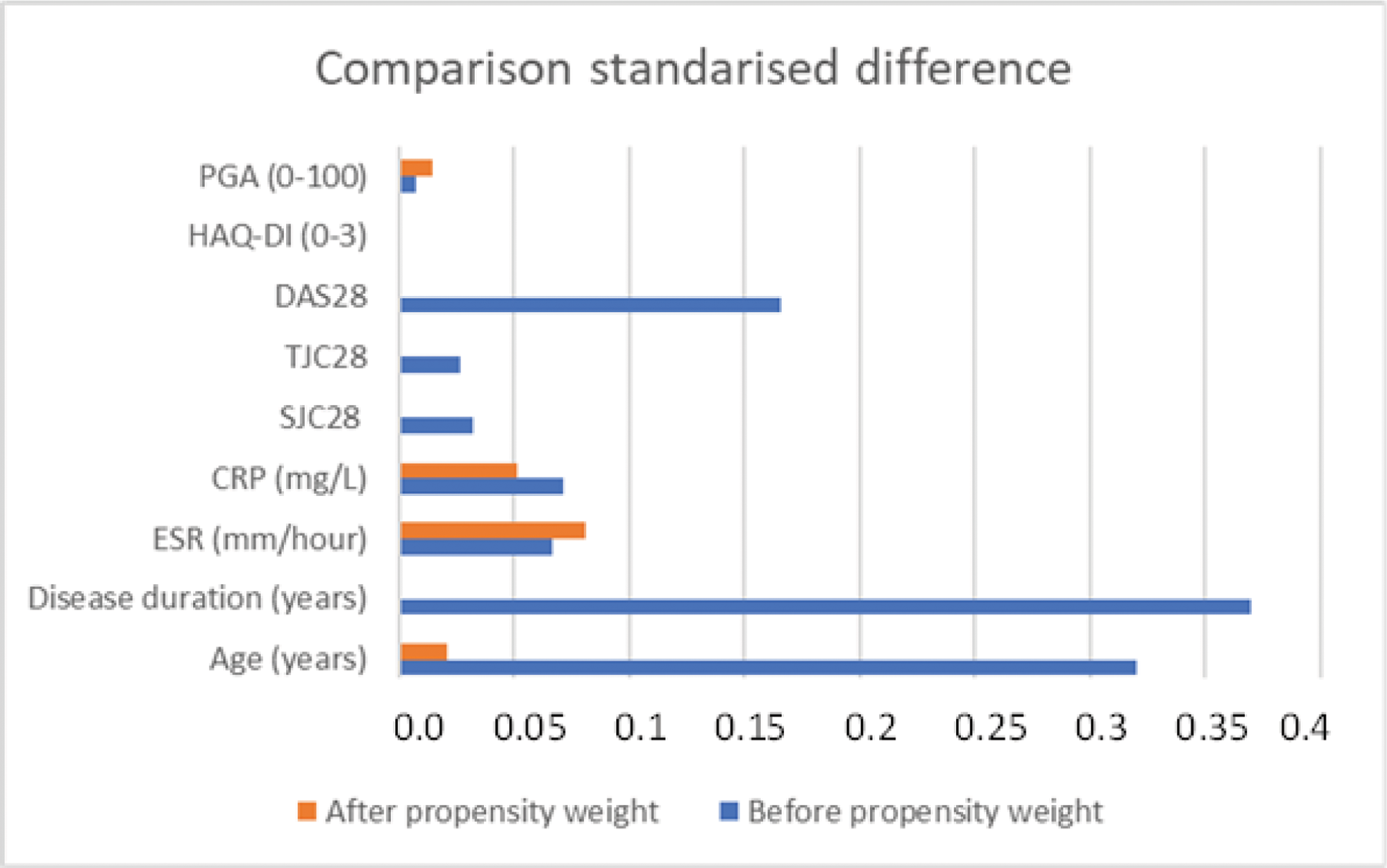
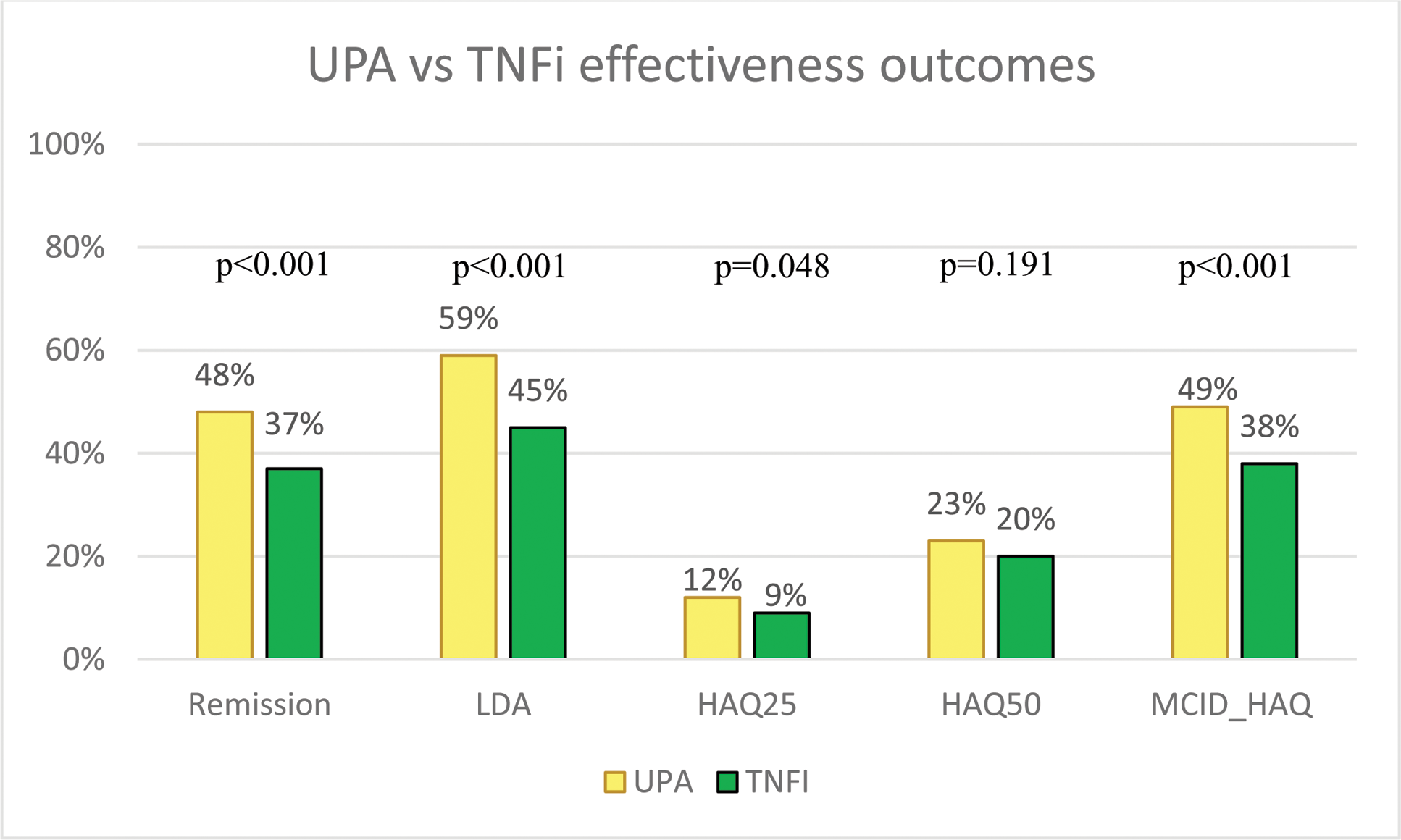
Results: In total, 610 patients on UPA and 915 patients on TNFi were included. Table 1 details these two populations. Figure 1 shows the standardised differences between groups of variables used in the propensity score before and after weighing. Remission was reached in 48.0% (293/610) of UPA and 37.0% (338/915) of TNFi patients (p<0.001). Proportion of patients reaching LDA or MCID_HAQ was also higher for UPA, while HAQ25 and HAQ50 were comparable (Figure 2). The propensity adjusted logistic regression models for UPA versus TNFi yielded relative risk ratios (95%CI) of 2.1 (1.7-2.6), 2.4 (1.9-2.9), 2.0 (1.4-2.8), 1.6 (1.3-2.0) and 2.0 (1.6-2.4) for remission (p<0.001), LDA (p<0.001), HAQ25 (p<0.001), HAQ50 (p<0.001) and MCID_HAQ (p<0.001) respectively. Finally, at the second visit in TARDIS, UPA was continued in 67.9% (414/610) of patients while TNFi was continued in 58.9% (539/915) of patients.
Conclusion: After accounting for baseline differences, UPA demonstrated greater real-world improvements on the long-term in disease activity and functional outcomes compared to TNF inhibitors in patients with moderate to severe RA.
REFERENCES: NIL.
Table 1. Baseline characteristics of TARDIS patients on UPA
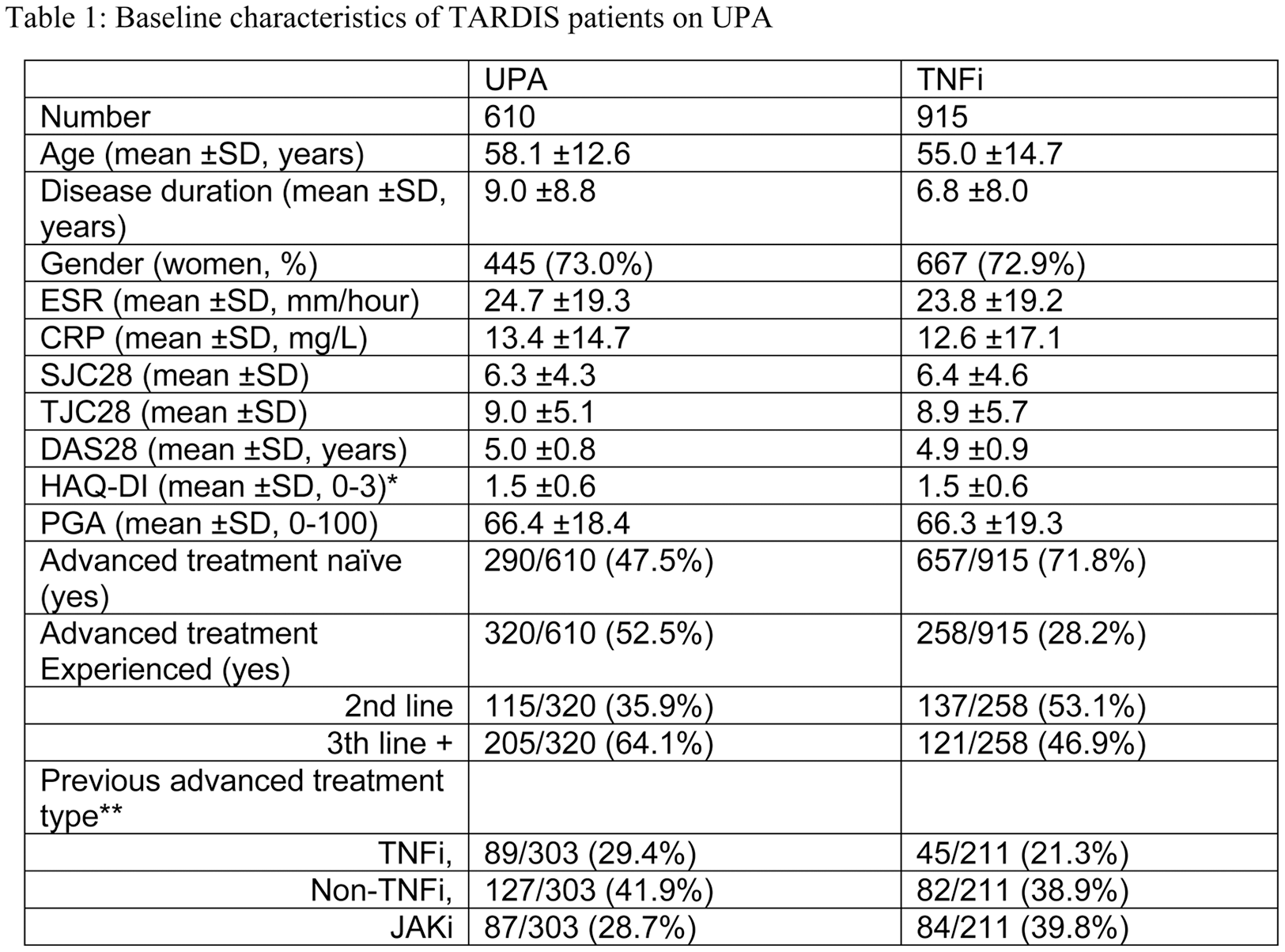
Legend: Number given are mean ± SD or number, proportion. TNFi = tumour necrosis factor inhibitor, JAKi = Janus Kinase inhibitor, HAQ= health assessment questionnaire, PGA= Patient Global assessment; CRP= C-reactive protein; ESR= erythrocyte sedimentation rate; TJC= tender joint count; SJC= Swollen joint Count; DAS28 = disease activity score based on the 28joints. Bionaive patients were patients starting UPA as first advanced therapy. *Missing HAQ(0-3) scores were imputed by regression using age and HAQ(0-60) scores. ** previous treatment was undefined.
Comparison of the standardised differences between UPA and TNFi on the variables used in the propensity before and after weighing. HAQ= health assessment questionnaire, PGA= Patient Global assessment; CRP= C-reactive protein; ESR= erythrocyte sedimentation rate; TJC= tender joint count; SJC= Swollen joint Count; DAS28 = disease activity score based on the 28 joints.
UPA vs TNFi comparative effectiveness outcomes at the time of the second visit in Tardis (27 months for UPA; 30 months for TNFi). Remission =DAS-28 < 2.6; LDA = Low disease activity (DAS-28 ≤ 3.2); HAQ25 = HAQ-DI score below 0.25; HAQ50 = HAQ-DI score below 0.5; MCID_HAQ = clinical meaningful HAQ-DI-decrease since baseline of 0.22. P-values generated by Chi-Square test between groups.
Acknowledgements: AbbVie sponsored the study and contributed to the design. AbbVie participated in the reviewing and approval of the final version. No honoraria or payments were made for authorship.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (