

Background: Current guidelines recommend treatment escalation to a biological (b) disease-modifying anti-rheumatic drug (DMARD) in patients with active rheumatoid arthritis (RA) failing methotrexate (MTX), at least among patients with poor prognostic factors such as anti-CCP positivity.
Objectives: To describe the type of DMARD used as second DMARD, and the response to this second DMARD, among patients who fail MTX as first ever DMARD in monotherapy.
Methods: Data from three European countries/centers were included: Region Stockholm (Sweden, SW), Leiden (Netherland, NL), and Oslo (Norway, NO). We included all RA patients at each center who had failed MTX in monotherapy as their first ever DMARD and started a second DMARD during the study period 2008-2023 (SW), 2011-2024 (NL), and 2001-2024 (NO, first line MTX only available in 2001-2012). The second DMARDs were categorised as other conventional synthetic DMARD (csDMARD: hydroxychloroquine, sulfasalazine, leflunomide, cyclosporine, azathioprine), tumor necrosis factor inhibitors (TNFi: adalimumab, infliximab, etanercept, certolizumab pegol, golimumab), non-TNFi (abatacept, anakinra, rituximab, tocilizumab, sarilumab), and Janus Kinase inhibitors (JAKi: tofacitinib, baricitinib, upadacitib, filgotinib). Further, DMARDs were categorised as switch or add-on to MTX after its failure, based on the discontinuation (or not) of MTX at the start of the second DMARD. Patients adding a DMARD to MTX within 30 days from MTX start were considered as starting a combination therapy as first-line treatment and excluded from these analyses. We analyzed survival on the second DMARD as the time from start of the second DMARD until start of a third DMARD, visualized via Kaplan-Meier curves. Stop of the second DMARD was not considered a censoring event. Retention at 12 months was defined as remaining on the second DMARD at 12 months without the introduction of a third DMARD. Remission at 6 and 12 months was defined as DAS28ESR<2.6 and was presented as the percentage among those with available information (no imputation of missing was performed). If a third DMARD was introduced before the 6- or 12-month time point, remission was imputed as not reached.
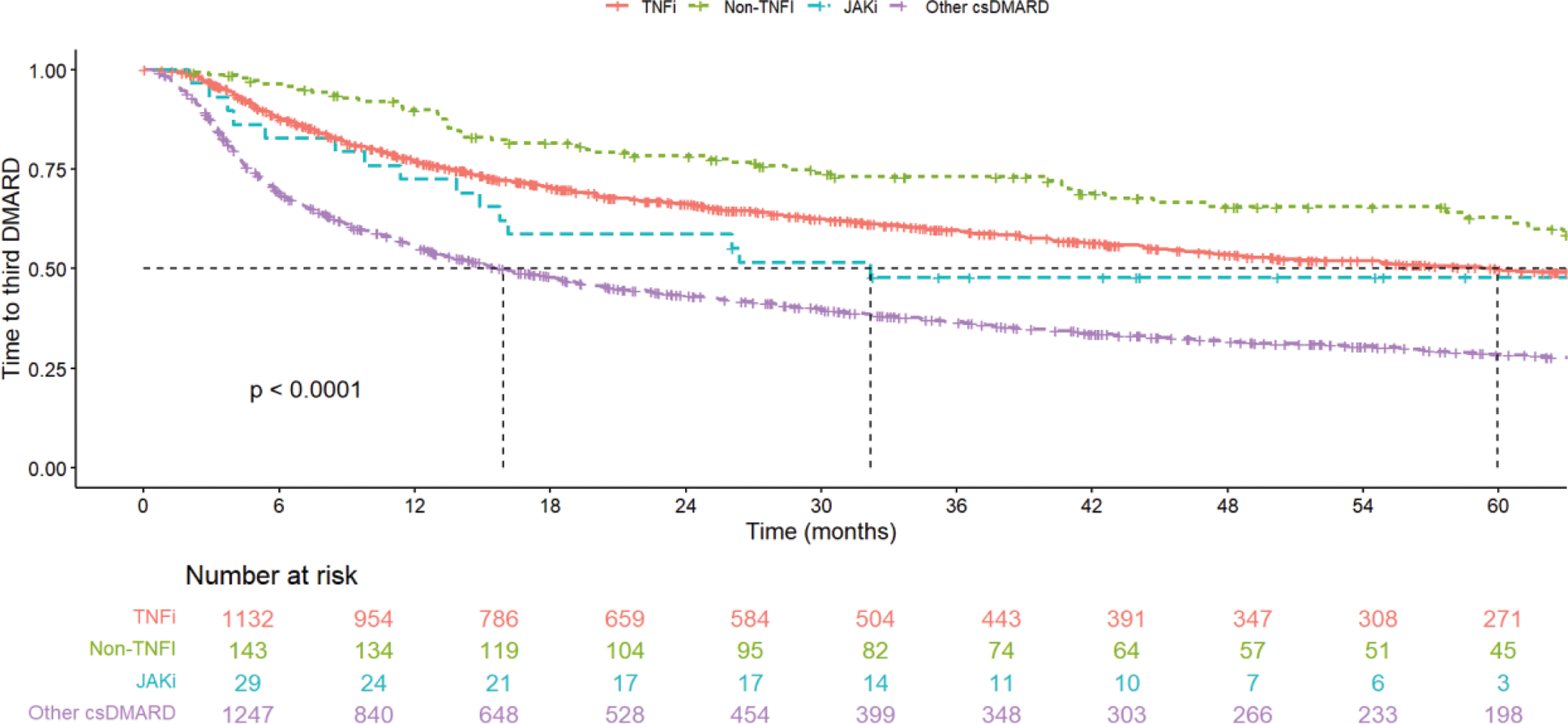
Results: In total, 2581 RA patients failed their first ever MTX in monotherapy and started a second DMARD (1509 SW, 611 NL, 461 NO). Most patients started a second csDMARD (49%), followed by TNFi (44%), while only 1% started a JAKi. There was a difference in the prevalence of the type of second DMARD between countries: SW and NO started mainly TNFi (72%, 53%), while NL started mainly other csDMARDs (95%). TNFi was most often used as add-on to MTX (62%), while treatment with other csDMARDs was mainly started after discontinuation of MTX (58%). Age was lower among TNFi users, while JAKi had the highest percentage of females (79%). Those using TNFi as second DMARD were also more often ACPA positive as compared to those using other csDMARD as second DMARD. Patients treated with TNFi and non-TNFi had a higher probability of being in retention at 12 months (69%/68%), compared to the JAKi (59%) and other csDMARD (40%). Looking at time to start of third DMARD, patients treated with csDMARDs as second DMARD(s) started a third DMARD eariler compared to the other second DMARD regimens, while patients treated with non-TNFi started a third DMARD later in time (Figure 1). The highest remission at 6/12 months was observed for JAKi (39%/57%), while TNFi and other csDMARDs had similar remission (29%/33% and 29%/33% respectively), and non-TNFi had the lowest remission rate (18%/27%). Treatments added to MTX had in general better retention and remission than treatment started after MTX discontinuation.
Conclusion: Patients starting a TNFi as second DMARD after failing MTX had better retention but similar percentage of remission as compared to other DMARD regimens used as second DMARDs. Further analyses are needed to understand how these differences are affected by confounding by indication.
REFERENCES: NIL.
Disease characteristics, remission and retention rates of patients starting the 2 nd DMARD after MTX failure
| TNFi | Non-TNFi | JAKi | Other csDMARDs | |
|---|---|---|---|---|
| N (% ) | 1146 (44) | 146 (6) | 29 (1) | 1260 (49) |
| Added to MTX, n (% ) | 709 (62) | 84 (58) | 14 (48) | 535 (42) |
| Age | 52 (14) | 59 (14) | 57 (14) | 58 (15) |
| Female, n (% ) | 837 (73) | 101 (69) | 23 (79) | 914 (73) |
| Acpa positive, n (% ) | 463 (76) | 61 (87) | 9 (69) | 548 (64) |
| Mean year of start | 2015 | 2016 | 2019 | 2016 |
| Country | ||||
| NL (% within country ) | 20 (3) | 11 (2) | <5 | 579 (95) |
| NO (% within country ) | 331 (72) | 21 (5) | <5 | 107 (23) |
| SW (% within country ) | 795 (53) | 114 (8) | 26 (2) | 574 (38) |
| Retention at 12 months (% ) | ||||
| DMARD not added to MTX | 68.3 | 70.5 | 46.7 | 36.5 |
| DMARD added to MTX | 69.2 | 66.7 | 71.4 | 42.3 |
| Remission* at 6 months (% ) | ||||
| DMARD not added to MTX | 26.5 | 20.7 | <5 | 28.8 |
| DMARD added to MTX | 29.4 | 16.3 | <5 | 28.2 |
| Remission at 12 months (% ) | ||||
| DMARD not added to MTX | 29.2 | 30.8 | <5 | 33.7 |
| DMARD added to MTX | 33.9 | 25.5 | <5 | 31.5 |
*DAS28 ESR <2.6
Kaplan Meier of time until switch to third DMARD.
Acknowledgements: This project has received funding from Horizon Europe programme under grant agreement no. 101095052 (SQUEEZE) and no. 101080711 (SPIDERR). We thank David Steeman for his help in extracting and processing the electronic health record data.
Disclosure of Interests: Daniela Di Giuseppe: None declared, Nils Steinz: None declared, Joe Sexton: None declared, Claudia Anna Hana: None declared, Helga Lechner-Radner: None declared, Daniela Sieghart: None declared, Sella Aarrestad Provan: None declared, Rachel Knevel: None declared, Johan Askling agreements between Karolinska Institutet (with JA as PI) and the listed entities, mainly for the national safety monitoring of rheumatology immunomodulators in Sweden (ARTIS): Abbvie, BMS, Eli Lilly, Galapagos, MSD, Pfizer, Roche, Samsung Bioepis, Sanofi.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (