

Background: According to the recent EULAR/Pres Recommendations for Still’s disease (SD) management, the employment of bDMARDs, in particular IL-1 inhibitors (i) is crucial to prevent flares, reduce the glucocorticoids intake (GC) and avoid the risk of disease progression [1].
Objectives: The objective of this study is to observe if patients with SD early treated with IL-1i achieve higher rates of clinical inactive disease (CID), compared to those late treated during the disease course, with either anakinra or canakinumab. We also assessed the recurrence of flares.
Methods: We gathered patients with SD (Yamaguchi’s criteria) diagnosed between 2010 and 2024. One-hundred and nine SD patients were screened; Excel data sheet was employed to collect clinical and laboratory data. CID was defined in accordance with the criteria provided for systemic juvenile idiopathic arthritis (sJIA) (Wallace Criteria) and adopted also for adults in the recent EULAR Recommendations [1, 2]. CID is defined as the absence of clinical manifestations of SD with normal levels of inflammatory markers at a single time point visit. CID achievement at six months (M) was set as primary outcome. Chi square test was employed as statistical test and p<0.05 was considered significant. GraphPad Prism8 was used for statistical analysis.
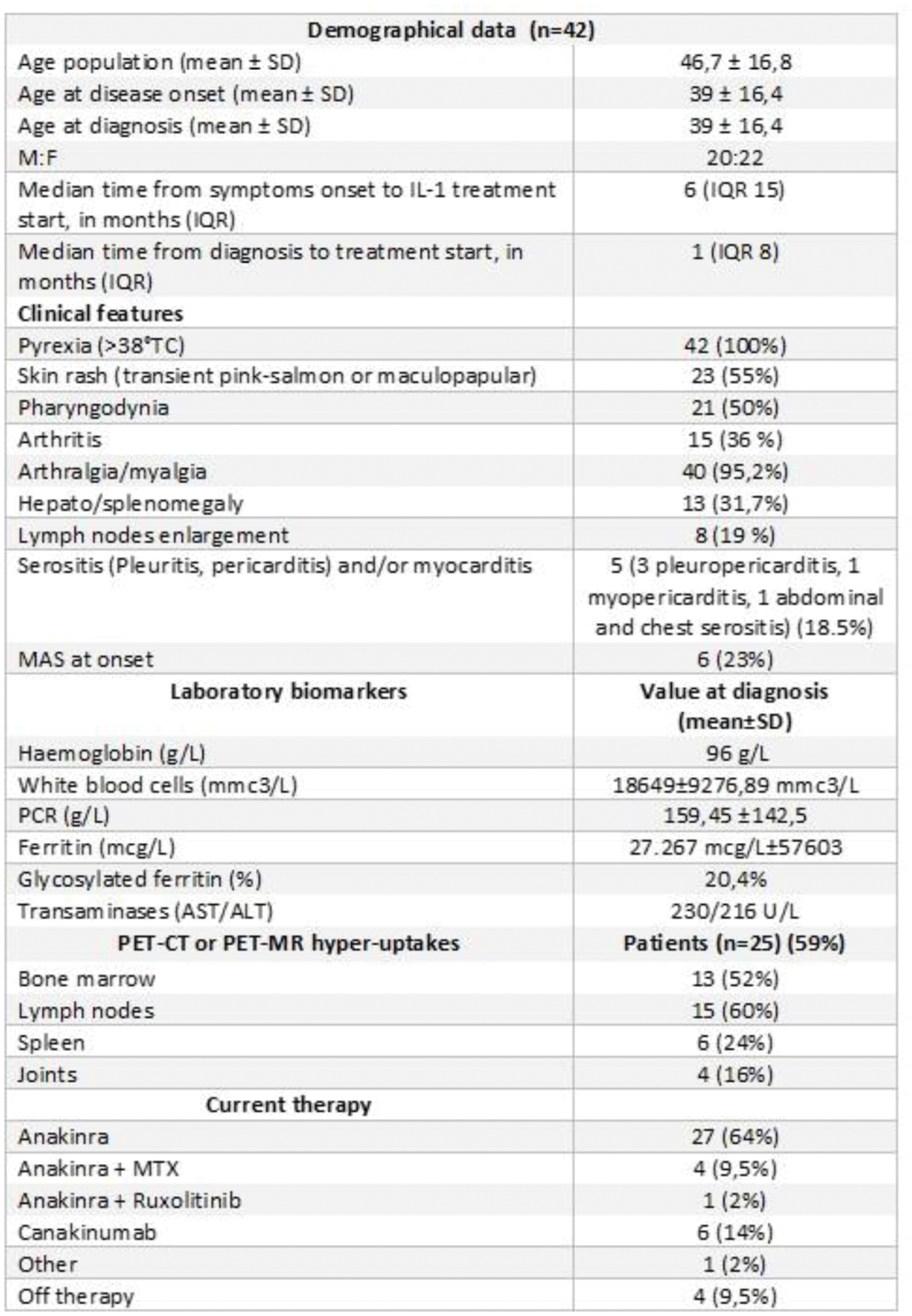
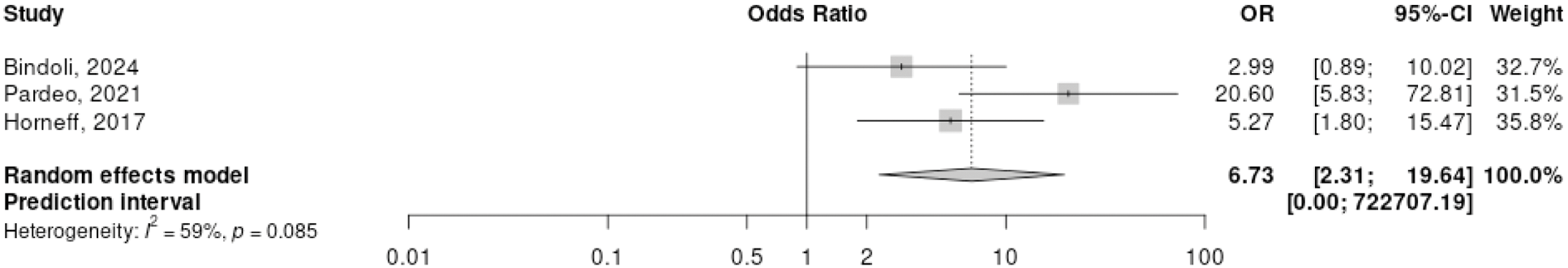
Results: We obtained data from 42 of the sixty-four patients with SD treated with IL-1i. Demographic, clinical, imaging and laboratory data are shown in Figure 1. Twenty-seven patients (64%) received the diagnosis within 6M after symptoms start. In 24/42 (57%), IL-1i was given within 6M from disease onset, whereas in 18 (43%) patients it was started >6M. Overall, CID at M6 was achieved by 23/42 (55%) patients; despite not significant (p=0.17), of the 24 patients that were early treated, 16 (67%) were in CID at M6, while of those treated later, CID was achieved by 7/18 (38%) at the fixed timepoint; a meta-analyses (Figure 2) carried out on two retrospective studies [3, 4] and the present study, comparing early vs. late IL-1i treated subgroups (the studies were heterogenous for timepoint of assessment and time of IL-1i introduction), showed an OR, to achieve CID, of 6.73 (95% IC 2.31 to 19.64); severe flares recurred after IL-1i discontinuation in three patients, while two had a refractory form. No serious adverse events (SAEs) were observed during IL-1i therapy.
Conclusion: Patients treated with IL-1i within 6M from onset had higher CID rates than those late-treated. Despite the significance was not achieved, our data are in line with those reported in other sJIA cohorts of IL-1i treated (3,4), which showed that early treatment (ranging between <3 and <12 M) was associated with higher CID remission rates than late treatment. Regarding safety, we did not observe SAEs related to IL-1i therapy and flares occurred mostly after IL-1i discontinuation.
REFERENCES: [1] Fautrel B, et al. EULAR/PReS recommendations for the diagnosis and management of Still’s disease, comprising systemic juvenile idiopathic arthritis and adult-onset Still’s disease. Ann Rheum Dis. 2024 Nov 14;83(12):1614-1627.
[2] Bindoli S, et al. Efficacy and safety of therapies for Still’s disease and macrophage activation syndrome (MAS): a systematic review informing the EULAR/PReS guidelines for the management of Still’s disease. Ann Rheum Dis. 2024 Nov 14;83(12):1731-1747.
[3] Pardeo M, Rossi MN, Pires Marafon D, et al. Early treatment and Il1Rn singlenucleotide polymorphisms affect response to anakinra in systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2021;73:1053–61.
[4] Horneff G, Schulz AC, Klotsche J, et al. Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry. Arthritis Res Ther 2017;19:256.
Clinical and demographic data of the SD patients evaluated
Forest Plot for CID achievement in early vs late IL-1i treated of three retrospective cohorts, including the present study
Acknowledgements: NIL.
Disclosure of Interests: Sara Bindoli: None declared, Cristina Cadore: None declared, Irina Guidea: None declared, Roberta Ramonda Takeda, Novartis, UCB, Andrea Doria GSK, Otsuka, Eli Lilly, Paolo Sfriso SOBI, Novartis.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (