

Background: Multicentric Castleman Disease (MCD) is a rare and heterogeneous lymphoproliferative disorder comprising a group of hematological conditions with overlapping histopathological and clinical features. It affects multiple lymph nodes and is characterized by systemic inflammation, cytopenias, and potentially life-threatening multiorgan dysfunction [1]. Multicentric cases negative for human herpesvirus-8 (HHV-8), representing 33% to 50% of multicentric cases, are considered idiopathic (iMCD)[2] after excluding other potential mimickers, such as autoimmune/autoinflammatory disorders and paraneoplastic cases. The clinical management of iMCD is challenging, requiring multidisciplinary collaboration between pathologists and clinicians. Diagnosis involves meeting specific clinical and pathological criteria while excluding other conditions [2]. Advances in treatment have highlighted the role of IL-6 in the pathogenesis of the disease [3]. The absence of consensus diagnostic criteria until 2017, lack of specific biomarkers [1], variability in response criteria and the disease’s low incidence have hindered physicians’ ability to gain experience and conduct clinical trials, making it challenging to evaluate treatment efficacy. Furthermore, the overlap with malignant, infectious, or autoimmune diseases and syndromes, combined with the heterogeneous clinical presentation of Castleman disease (CD), likely lead to a significant proportion of CD patients being misdiagnosed or remaining unrecognized. iMCD, as a potentially life-threatening systemic disease, represents an important differential diagnosis in rheumatological practice [4]. All of this contributes to an unfavorable prognosis of iMCD patients, with a mortality rate of 35% at 5 years and 60% at 10 years from diagnosis [5]. Prevalence data on iMCD is limited, and primarily comes from outside Europe. In 2015, the estimated incidence rate was 5 patients per million/year, with regional variations [6, 7]. Limitations of this data include the lack of a formal disease definition, variability in incidence estimates, and the exclusion of patients with certain conditions, which may lead to an underestimation of the actual incidence of the disease [8]. Epidemiologic studies of rare diseases are vital to assess their impact, uncover etiological factors, promote targeted therapies, and provide robust future estimates. To address these gaps, the Spanish iMCD Registry was proposed.
Objectives: The Spanish iMCD Registry is an observational, multicenter, prospective study designed to improve the understanding of the epidemiology, clinical features, and treatment outcomes of iMCD in Spain. This study seeks to provide valuable real-world data on demographics, pathology, symptoms, treatments, and outcomes, contributing to more robust global estimates in the future.
Methods: This study aims to enroll approximately 60 patients from 20 centers across Spain. It comprises two cohorts (Figure 1):
Prevalence cohort: Historical cases of patients with compatible iMCD diagnosis, analyzed to describe clinical characteristics and treatment patterns.
Incidence cohort: Newly diagnosed iMCD patients identified during the recruitment period, who will be followed prospectively.
Spanish iMCD Registry: Study Design Overview
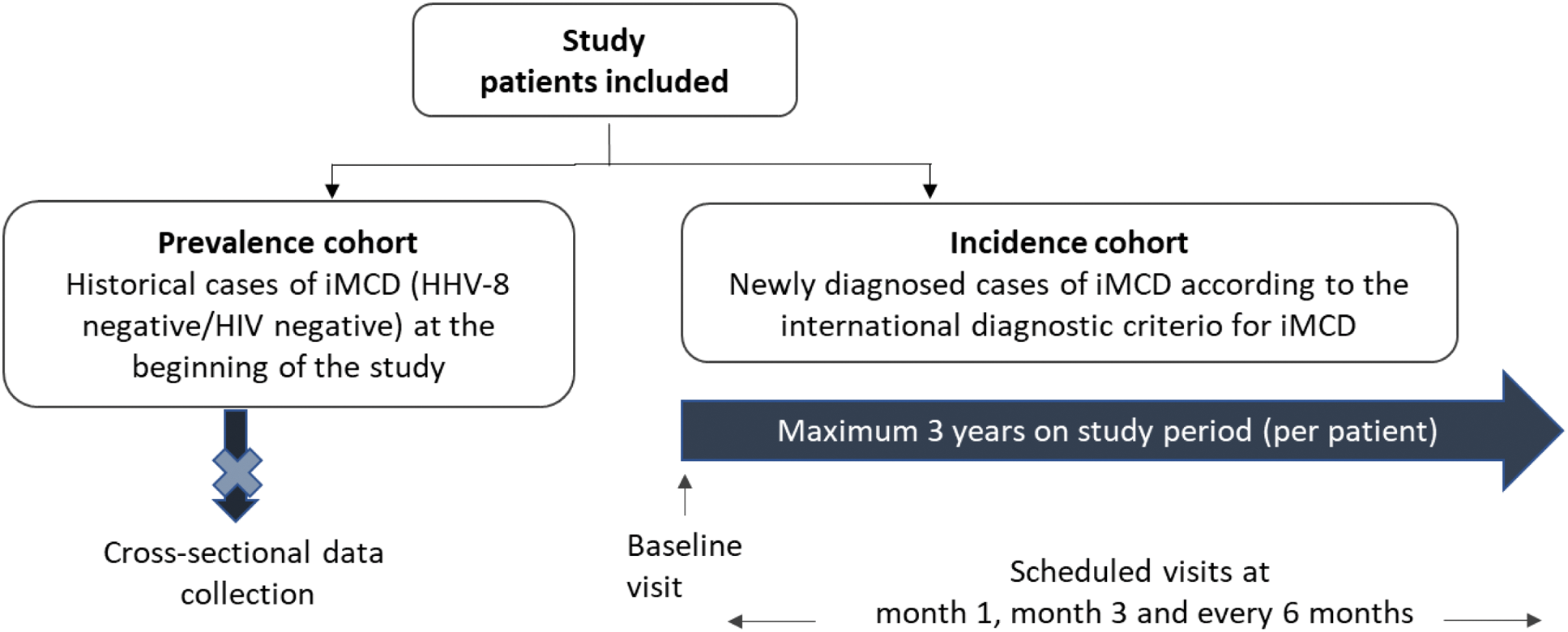
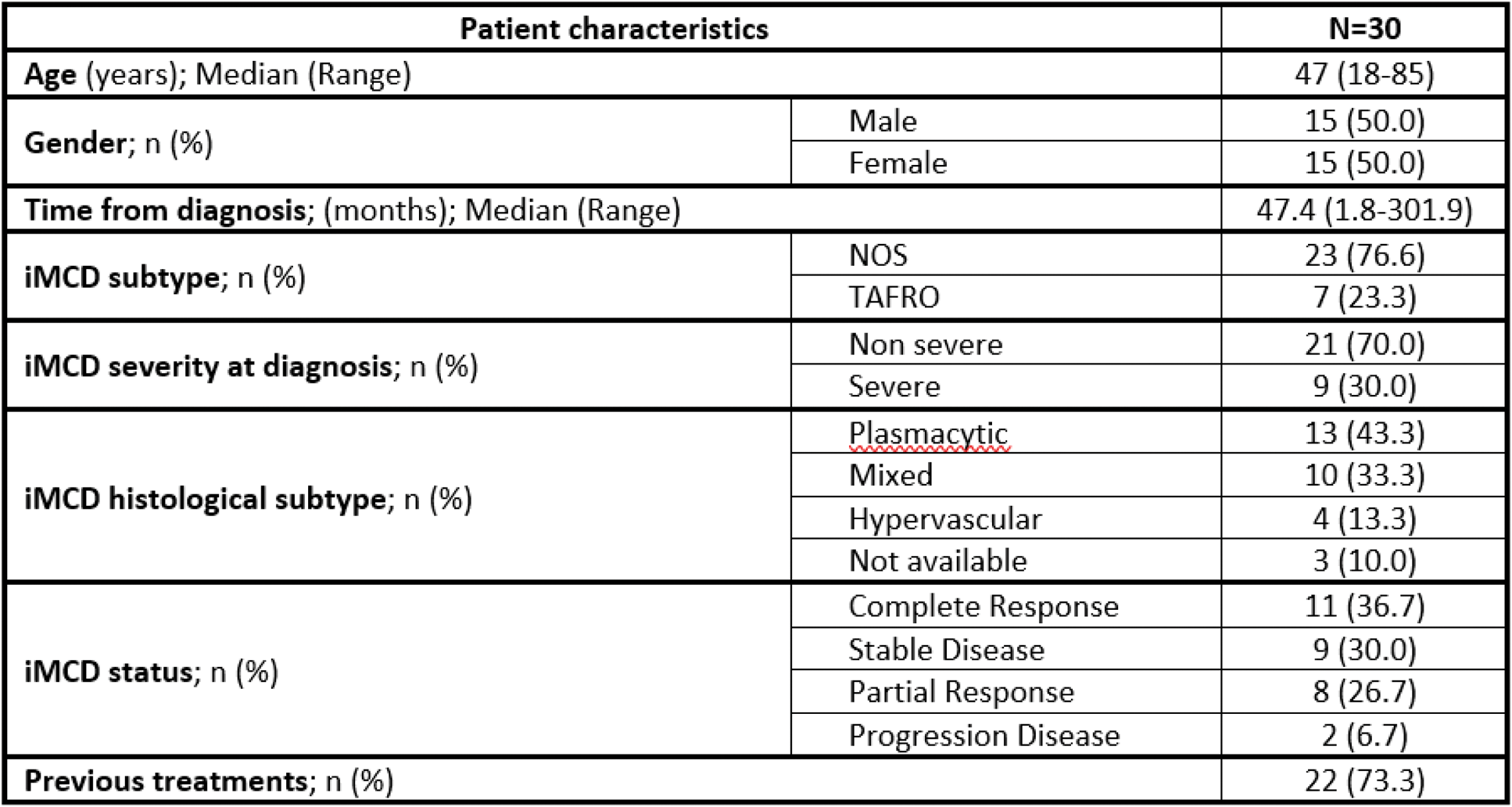
Results: As of January 2025, 41 patients have been enrolled in the study, with 33 meeting the inclusion criteria: 30 in the prevalence cohort and 3 in the incidence cohort. The remaining 8 individuals (7 from the prevalence cohort and 1 from the incidence cohort) did not meet the diagnostic criteria and were classified as screening failures. Preliminary results from the prevalence cohort include 30 patients across 15 Spanish hospitals. The median age was 47 years, with an equal gender distribution and 76.7% being Caucasian. Disease characteristics are summarized in Table 1. At the time of study entry, 73.3% of individuals had undergone prior treatments, with a median of 2 treatment lines (range 1-7). Currently, 21 patients remain on treatment, with siltuximab being the most common therapy (85.7%), followed by sirolimus (4.8%) and tocilizumab (4.8%). The estimated prevalence of iMCD was 0.50715 cases per 100.000 inhabitants (95% CI: 0,32567-0,68863). Comorbidities were observed in 70% of patients (n=21). Among these, 23.8% involved autoimmune or autoinflammatory diseases, including conditions such as thrombotic thrombocytopenic purpura, psoriasis, immune thrombocytopenia, Graves’ disease, autoimmune hemolytic anemia, and acquired hemophilia.
Table 1. Patient characteristics at diagnosis (N=30)
Conclusion: This is the first study to assess the prevalence of iMCD in Spain. The reported prevalence aligns with previously published results. The IL-6 blockade is the most commonly used therapeutic approach in the cohort. These findings provide essential insights into the epidemiology and management of iMCD, contributing to the global understanding of this rare disease.
REFERENCES: [1] Fajgenbaum DC. Blood 2018;132:2323-2330.
[2] Fajgenbaum DC, et al. Blood 2017;129:1646-1657.
[3] van Rhee F, et al. Lancet Haematol 2020;7:e209-e217.
[4] Schmalzing M, et al. Z Rheumatol 2024;83(3):289-298.
[5] Dispenzieri A, et al. Am J Hematol 2012;87:997-1002.
[6] Munshi N, et al. Leuk Lymphoma 2015;56:1252-1260.
[7] Simpson D. Hematol Oncol Clin North Am 2018;32:1-10.
[8] Mukherjee S, et al. Blood Adv 2022;6(2):359-367.
Acknowledgements: NIL.
Disclosure of Interests: Andrés González García GSK, Otsuka, AstraZeneca, Recordati Rare Diseases, Sobi, GSK, Otsuka, Recordati Rare Diseases, Pedro Durán del Campo Recordati Rare Diseases, Rosario Sánchez Martínez Chiesi, Shire, Takeda, Amicus Therapeutics, Sanofi-Genzyme, EusaPharma (Recordati Rare Diseases), Alnylam, Pfizer, Bayer, José-Tomás Navarro Recordati Rare Diseases, Novartis, Recordati Rare Diseases, Gilead, Santiago Montes-Moreno Recordati Rare Diseases, Roche y Janssen, Gilead (2019).
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (