

Background: Current treatments for osteoarthritis of the knee (OAK) are limited and provide only temporary pain relief [1]. PCRX-201 is a high-capacity, nonintegrating, nonreplicating adenovirus serotype 5 vector for intra-articular (IA) injection in patients with OAK [2]. In response to local inflammation and under control of an inducible promotor, PCRX-201 expresses interleukin-1 receptor antagonist, which is an inhibitor of interleukin-1 signaling [2]. Data suggest that PCRX-201 could potentially lead to reduced long-term pain and disability.
Objectives: The objective of this open-label phase 1 trial (NCT04119687) was to investigate the safety and efficacy of PCRX-201 for up to 260 weeks (5 years). The 104-week data were previously presented, and the current updated analysis reflects follow-up to 156 weeks (3 years).
Methods: Participants aged 30-80 years (N=72) with OAK (Kellgren/Lawrence [K/L] grade 2-4) were enrolled. The first cohort received ultrasound-guided IA injection of PCRX-201 in the target knee at 1 of 3 doses (n=36). The second cohort received pretreatment with IA methylprednisolone 40 mg immediately before PCRX-201 administration at the same doses (n=36) to explore the benefit of immune modulation to maximize vector tolerability and transduction. Outcomes up to 156 weeks included safety, WOMAC pain (WOMAC-A) and stiffness (WOMAC-B) scores, and the Knee Injury and Osteoarthritis Outcome Score (KOOS).
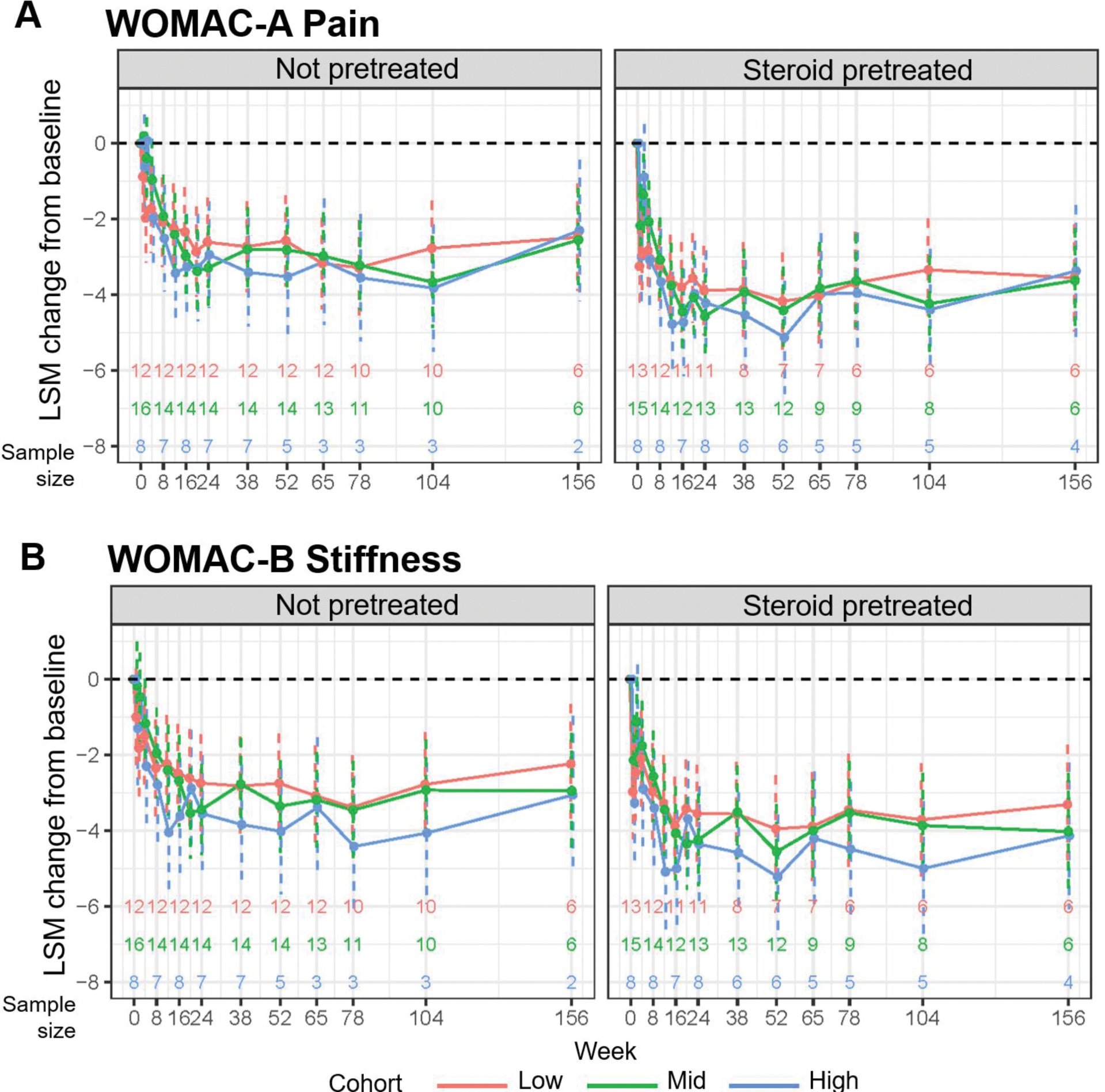
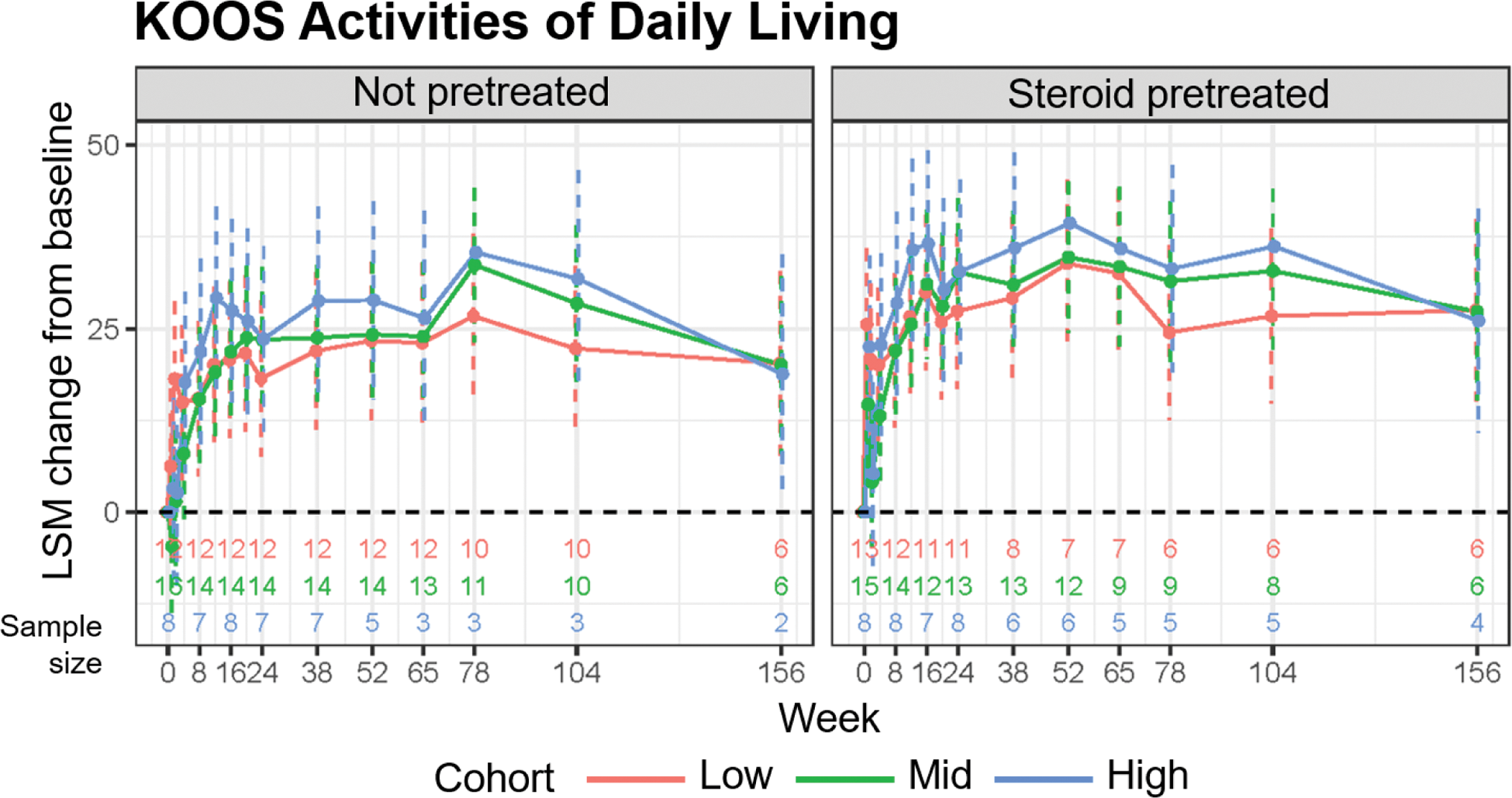
Results: In each cohort, 36 participants were enrolled and treated. Participants in the not pretreated cohort had a median age of 62.0 years and those in the steroid pretreated cohort had a median age of 67.5 years. Most participants were female in both cohorts (59%) with a K/L grade of 3 to 4 (81% in the not pretreated group and 83% in the steroid pretreated group). Mean WOMAC-A pain score was 6.8 in both cohorts, and ~75% of participants had bilateral OAK. No procedure- or treatment-related serious adverse events (AEs) were reported. Dose-related index knee effusion was the most commonly reported AE and occurred less frequently in the steroid pretreated cohort (13/36 [36%]) than the not pretreated cohort (22/35 [63%]) as observed. At 156 weeks, 14 participants remained in the not pretreated group (9 discontinued between weeks 104 and 156) and 16 remained in the steroid pretreated group (3 discontinued between weeks 104 and 156). No additional dose-related index knee effusions were reported between 104 and 156 weeks. At all doses and across both cohorts, pain and function benefits were observed, with the greatest improvements occurring in the steroid pretreated cohort (Figure 1); subsequent results focus on this cohort. In the steroid pretreated cohort across doses, the least squares mean (LSM) improvement from baseline for WOMAC-A score at 156 weeks was 3.4-3.6 points (51%-53% reduction from baseline pain as observed; Figure 1A, right panel). WOMAC-A scores between 104 and 156 weeks for higher doses in the steroid pretreated cohort exhibited a larger LSM change from baseline compared with lower doses. LSM improvements from baseline were also observed for WOMAC-B score at 156 weeks (3.3-4.1 points [38%-76% reduction from baseline stiffness as observed]; Figure 1B, right panel). LSM improvements from baseline in KOOS activities of daily living scores were observed at 156 weeks (26.1-27.5 [of 100] points) for the steroid pretreated cohort across doses (Figure 2).
Conclusion: A single IA injection of PCRX-201 in the index knee had an acceptable safety profile with improvements in pain, function, and stiffness sustained through 156 weeks, indicating long-term clinical efficacy. The sustained improvements in WOMAC pain and stiffness scores at 156 weeks suggest that lower doses of PCRX-201 may be sufficient to provide efficacy in future clinical studies. Study follow-up is ongoing through 260 weeks. Given these data, steroid pretreatment will be used in randomized, double-blind, active-controlled phase 2 studies planned for initiation in 2025.
REFERENCES: [1] Buelt and Narducci. Am Fam Physician . 2021;103:120-121.
[2] Senter et al. Hum Gene Ther . 2022;33:541-549.
LSM change from baseline for (A ) WOMAC-A pain and (B ) WOMAC-B stiffness across 3 doses in the not pretreated and steroid pretreated cohorts through week 156. LSM, least squares mean; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
LSM change from baseline in KOOS activities of daily living scores across 3 doses in the not pretreated and steroid pretreated cohorts through week 156. KOOS, Knee Injury and Osteoarthritis Outcome Score; LSM, least squares mean.
Acknowledgements: NIL.
Disclosure of Interests: Philip G. Conaghan AbbVie, Janssen, Novartis, Sandoz, Alfasigma, Eli Lilly, Eupraxia, Formation Bio, Genascence, GSK, Grunenthal, Janssen, Kolon TissueGene, Levicept, Medipost, Moebius, Novartis, Stryker, Takeda, Ali Mobasheri HALEON, Janssen Cilag (Brazil), Viatris, Consumer Healthcare at Sanofi (France), Laboratoires Expansciences, HALEON (Global Pain Faculty, Naturals Advisory Board), Sanofi, Sanofi Consumer Healthcare (Opella Healthcare), Kolon TissueGene, Enlivex, Pacira BioSciences, Contura, Chondrometrics, Aptissen SA, Synartro AB, Contura AB, ICM (South Korea), Kangstem, Peptinov, Pluri, Chondropeptix, Infinity Research Labs, and the California Institute for Regenerative Medicine (CIRM), California’s Stem Cell Agency. I have received grants from Merck Serono, Stanley Cohen Lifordi, Lifordi, Pacira, Abbvie, Lilly, Pfizer,Spyre,UCB, Marc C. Hochberg Regenosine and Theralogix LLC, Pacira BioScience, Alan Kivitz GSK, Eli Lilly, Pfizer, UCB, Sanofi - Regeneron, Pfizer, GSK, Gilead, Novartis, Amgen, Abbvie, Coval, Ecor1, Fresenius Kabi, Gilead, Grunthal, GSK, Halia, Horizon, Innovaderm, Janssen, Moonlake, Pacira, Prometheus, Santa Ana Bio, Inc., Synact, Takeda - Numbus, UCB, VYNE, XBiotech, Xencor, Nino Joy Pacira BioSciences, Inc., Pacira BioSciences, Inc., Derek Jackson Pacira BioSciences, Inc., Pacira BioSciences, Inc., Masato Nakazawa Pacira BioSciences, Inc., Pacira BioSciences, Inc., Mary DiGiorgi Pacira BioSciences, Inc., Pacira BioSciences, Inc., Jonathan Slonin Pacira BioSciences, Inc., Pacira BioSciences, Inc.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (