

Background: The efficacy of secukinumab is well established in long-term clinical trials [1]. However, real-world evidence on the long-term use of secukinumab is limited. SERENA is a large, longitudinal, observational study conducted at 438 sites across Europe in adult patients with moderate to severe chronic plaque psoriasis, active psoriatic arthritis (PsA) and radiographic axial spondyloarthritis [2].
Objectives: To evaluate the 5-year effectiveness of secukinumab treatment in controlling disease activity in patients with PsA.
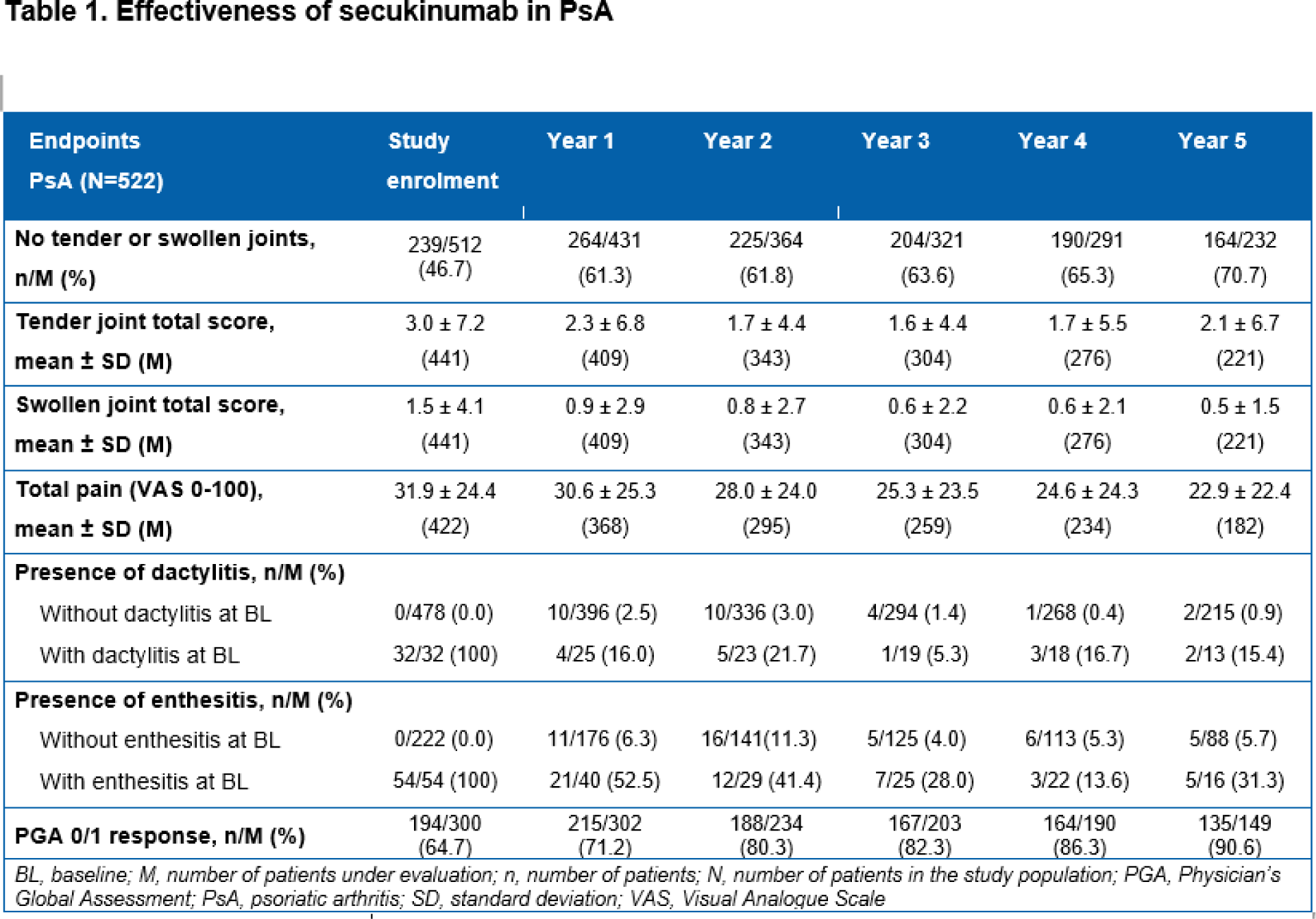
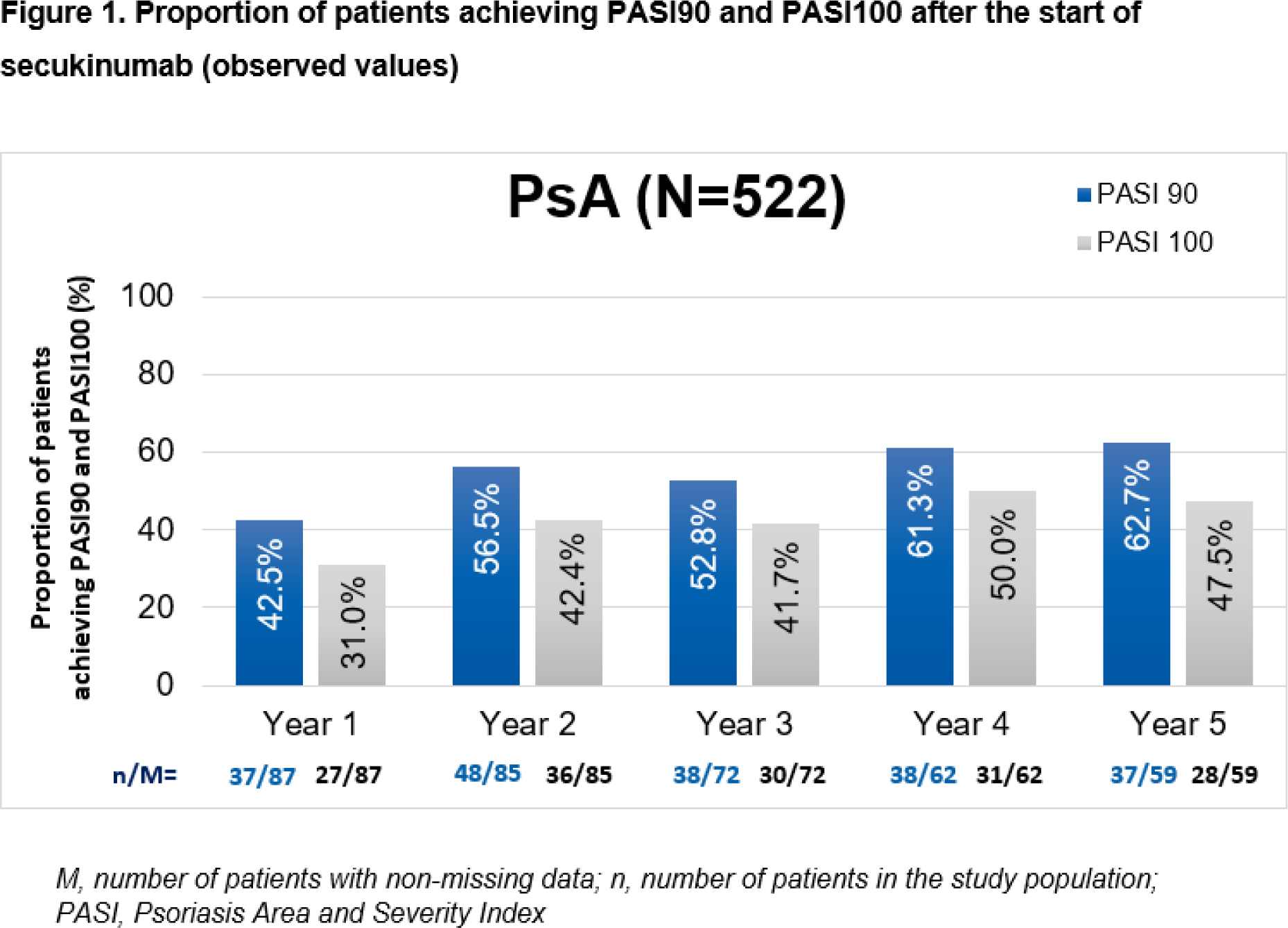
Methods: Patients received secukinumab treatment for ≥16 weeks before enrolment into the study. The effectiveness of secukinumab was evaluated after inclusion in the study based on 1) the proportion of patients reporting tender or swollen joints and their corresponding total scores; 2) total pain as assessed by Visual Analogue Scale (0-100); 3) the evolution of dactylitis and enthesitis; 4) skin clearance as assessed by the proportion of patients with Physician’s Global Assessment (PGA 0/1 [clear/almost clear skin]) response and those achieving Psoriasis Area and Severity Index 90/100 (PASI 90 or PASI100) after the start of secukinumab. Descriptive statistics of effectiveness assessments were based on observed data.
Results: Overall, 522 patients with active PsA were included in the analysis (mean age at study enrolment, 52.5 years; male, 44.8%; Caucasian, 94.1%; mean body mass index, 28.7 kg/m 2 ; mean time since diagnosis, 8.6 years). Most patients (68.6%) had previous biologic exposure before the start of secukinumab. After 5 years of study enrolment, most patients (70.7%) remained free from tender or swollen joints. The mean total scores of tender joints decreased from 3.0±7.2 at study enrolment to 2.1±6.7 by the end of Year 5. Similarly, the mean total scores of swollen joints decreased from 1.5 ± 4.1 to 0.5 ± 1.5 at the end of Year 5 (Table 1). Mean change from study enrolment in tender joint total scores was −0.3 ± 8.7 by Year 1 and −0.5 ± 8.4 by Year 5. Similarly, mean change from study enrolment in swollen joint scores was −0.3 ± 4.3 by Year 1 and −0.6 ± 4.1 by the end of Year 5. Total pain scores also decreased from the time of enrolment (31.9 ± 24.4) to Year 5 (22.9 ± 22.4; Table 1). At enrolment, the presence of dactylitis and enthesitis was reported in 32 and 54 patients, respectively. By the end of Year 5, only a small proportion of the patients with baseline dactylitis or enthesitis still had dactylitis (2 of 13 [15.4%]) and enthesitis (5 of 16 [31.3%]) respectively, and the proportion of patients developing new dactylitis was 0.9% and those developing new enthesitis was 5.7% (Table 1). Secukinumab treatment resulted in a sustained control over psoriatic skin changes as assessed by PGA 0/1 response, with above 90% of patients with skin clearance at the end of Year 5 (Table 1). At the end of 5 years, approximately 63% (37/59) and 48% (28/59) of patients remained PASI 90 and PASI100, respectively (Figure 1). The mean change in total PASI scores after 5 years from start of secukinumab was -9.18 ±14.55.
Conclusion: SERENA is one of the largest real-world studies in Europe. Long-term secukinumab treatment in patients with PsA resulted in control of the evolution of dactylitis and enthesitis and various other measures of disease activity including joint pain. Secukinumab-treated patients also achieved sustained long-term skin clearance.
REFERENCES: [1] Blair HA. Secukinumab: A review in psoriatic arthritis. Drugs 2021;81(4): 483-494. doi: 10.1007/s40265-021-01476-3.
[2] Kiltz U, Sfikakis PP, Gaffney K, Bounas A, Gullick N, Lespessailles E, Brandt-Juergens J, Rashkov R, Schulz B, Pournara E, Jagiello P. Interim 2-year analysis from SERENA: A real-world study in patients with psoriatic arthritis or ankylosing spondylitis treated with secukinumab. Rheumatol Ther. 2022;9(4): 1129-1142. doi: 10.1007/s40744-022-00460-x.
Acknowledgements: NIL.
Disclosure of Interests: Uta Kiltz For AbbVie, Amgen, Biogen, Chugai, Eli Lilly, Fresenius, Gilead, GSK, Grünenthal, Hexal, Janssen, MSD, Novartis, Pfizer, Roche and UCB, AbbVie, Amgen, Biogen, Chugai, Eli Lilly, Fresenius, Gilead, GSK, Grünenthal, Hexal, Janssen, MSD, Novartis, Pfizer, Roche and UCB, Petros P. Sfikakis AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis and Pfizer, AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, Novartis and Pfizer, Andreas Bounas AbbVie, Aenorasis, Amgen, Bausch Health, FARAN, Genesis Pharma, GSK, Janssen, MSD, Novartis, Pfizer and UCB, Nicola Gullick AbbVie, Eli Lilly, Janssen, Novartis and UCB. Consultant for AbbVie, Alfasigma, Janssen, Novartis and UCB, AbbVie, AstraZeneca, Eli Lilly, Janssen, Novartis and UCB, Eric Lespessailles AbbVie, Amgen, Expanscience, Galapagos, Lilly and MSD, Amgen, Expanscience, Eli Lilly and MSD, AbbVie, Amgen, Eli Lilly, MSD, Novartis and UCB, Jan Brandt-Juergens AbbVie, Pfizer, Roche, Sanofi-Aventis, Novartis, Eli Lilly, MSD, UCB, BMS, Janssen and Medac, AbbVie, Pfizer, Roche, Sanofi-Aventis, Novartis, Eli Lilly, MSD, UCB, BMS, Janssen and Medac, Rasho Rashkov AbbVie, Amgen, Pfizer, Novartis, MSD, UCB, Roche and Janssen, AbbVie, Amgen, Pfizer, Novartis, MSD, UCB, Roche and Janssen, Cynthia Vizcaya Employee of Novartis Pharma AG, Andreas Clemens Employee of Novartis Pharma AG, Barbara Schulz Employee of GKM Gesellschaft für Therapieforschung mbH, Lessingstrasse, München, Germany, and provides services to Novartis, Weibin Bao Employee of Novartis, Piotr Jagiello Former Employee of Novartis, Karl Gaffney AbbVie, Celgene, Eli Lilly, Pfizer, Gilead, MSD, Novartis and UCB, AbbVie, Celgene, Eli Lilly, Pfizer, Gilead, MSD, Novartis and UCB, AbbVie, Celgene, Eli Lilly, Pfizer, Gilead, MSD, Novartis and UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (