

Background: Programmed cell death protein-1 (PD-1) is an inhibitory receptor predominantly expressed on T cells, particularly on chronically activated effector and follicular helper/peripheral helper T cells. PD-1 antagonizing therapeutic antibodies in cancer therapy release an immune break, potentially leading to immune-related adverse events, including colitis and arthritis, in a subset of patients. Rheumatoid arthritis (RA) is characterized by marked PD-1 + T cell infiltration into inflamed synovium, suggesting dysregulated PD-1 activity contributes to disease pathogenesis. JNJ-67484703 is a humanized IgG1k antibody that agonizes and depletes PD-1 + T cells, potentially reducing aberrant inflammatory responses in autoimmune conditions.
Objectives: To evaluate the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and efficacy of JNJ-67484703 following multiple subcutaneous administrations in participants with active RA.
Methods: This was a Phase 1, randomized, double-blind, placebo-controlled, multiple-dose, multicenter study of approximately 30 weeks in duration, with a screening visit within 6 weeks prior to study drug intervention, 10 weeks of study treatment (at Weeks 0, 1, 2, 4, 6, 8, and 10), and 14 weeks of follow-up. Participants with active RA were randomly assigned to receive JNJ-67484703 2 mg/kg or placebo subcutaneously in a 5:1 ratio for the first 6 patients. Safety was assessed through Day 29, and it was determined that enrollment would proceed to the 3 mg/kg dose level. Cumulative safety and tolerability were assessed through Day 29 for the next 6 patients in a 5:1 ratio. The remaining 32 patients were enrolled to achieve an overall 2:1 JNJ-67484703 3 mg/kg:placebo ratio to assess efficacy. Key eligibility criteria were as follows: age18-65 years at enrollment, diagnosis of RA for >6 months based on American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria (2010), rheumatoid factor or anti-cyclic citrullinated peptide antibody positive, ≥6 swollen and ≥6 tender joint counts (screening and Day 1), and C-reactive protein (CRP) ≥0.3 mg/dL (screening). Participants were also required to have an inadequate response to ≥12 weeks of conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) and maintain stable doses of >1 csDMARDs during the study. Stable doses of non-steroidal anti-inflammatory drugs and oral glucocorticoids < 10 mg prednisone equivalent were also permitted. The primary objective to characterize the safety and tolerability of JNJ-67484703 was assessed by number of treatment-emergent adverse events (TEAEs) and other safety measures. Key secondary objectives of PK, immunogenicity, PD, and efficacy of JNJ-67484703 were also measured.
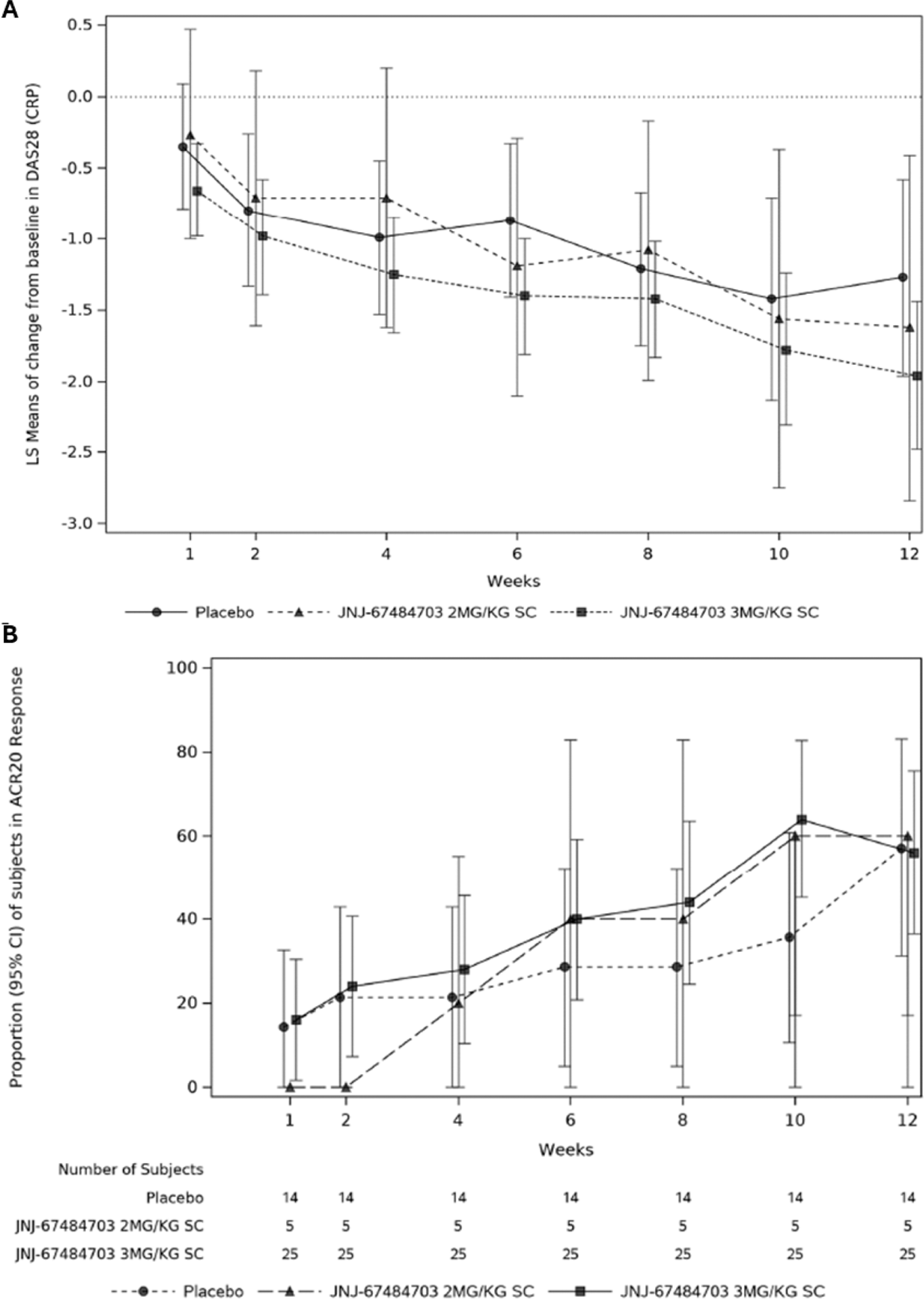
Results: Of 92 patients screened, 44 patients were randomized to JNJ-67484703 2 mg/kg (n=5), 3 mg/kg (n=25) or placebo (n=14). Demographics and baseline characteristics were comparable across the JNJ-67484073 and placebo groups, with a median age of 55.5 years, disease duration of 8.7 years, and baseline DAS-28-CRP of 6.3. All patients were receiving methotrexate, 72.7% were receiving oral glucocorticoids, and 40.9% were receiving NSAIDs. No participants had prior exposure to biologic or targeted-synthetic DMARDs. TEAEs were reported in 4 (80%) patients receiving JNJ-4703 2 mg/kg, 17 (68%) patients receiving JNJ-4703 3 mg/kg, and 10 (71%) patients receiving placebo. None of the reported TEAEs were severe in intensity, and no deaths were reported during the study. Three serious TEAEs were reported in 2 participants: SARS-COV-2 infection followed by pneumonia in 1 participant (2 mg/kg group) and community-acquired pneumonia in 1 participant (3 mg/kg group). Infections occurred in approximately 20% of patients in each treatment group, with urinary tract infections (UTIs) the most common (7.1% placebo, 10% combined JNJ-67484703 group). All patients who developed UTIs had a history of recurrent UTIs and no clinical signs of active infection at enrollment. At Week 12, the least squares (LS) mean difference (95% confidence interval (CI)) in Week 12 DAS28-CRP from baseline between the JNJ-67484703 3 mg/kg and placebo groups was -0.69 (-1.55, 0.18); nominal p=0.117 (Figure 1A). At the end of follow-up (Week 24), both JNJ-67484703 groups continued to show numerically greater reductions in DAS28-CRP versus placebo, but the magnitude of the difference decreased; the LS mean difference (95% CI) between JNJ-67484703 3 mg/kg and placebo was -0.47 (-1.59, 0.64) with nominal p=0.396. At Week 12, the proportions of patients achieving ACR20, ACR50, and ACR70 were generally comparable across treatment groups, but analysis by visit showed numerically greater ACR20 responses in the JNJ-67484703 3 mg/kg group versus placebo between Week 4 and Week 10 (Figure 1B). JNJ-67484703 induced a decrease in circulating PD-1 + CD4 + and CD8 + T cells that was dependent on the cell surface density of PD-1 expression. Steady state serum concentrations of JNJ-67484703 were approximately achieved by Week 4, and overall incidence of antibodies to JNJ-67484703 was low.
Conclusion: Overall, administration of JNJ-67484703 at 2 mg/kg and 3 mg/kg through 10 weeks was safe and well-tolerated. Numerically greater improvement in Week 12 DAS28-CRP from baseline was observed in the 3 mg/kg group compared to placebo. Participants treated with JNJ-67484703 showed a selective decrease in circulating PD-1 + T cells. The overall incidence of antibodies to JNJ-67484703 was low.
A ) Change from baseline in DAS28-CRP score and B ) proportion of patients achieving ACR20 response by-visit through Week 12 with treatment failure rules applied.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Irving Ling Johnson & Johnson, Johnson & Johnson, Stanley Marciniak Johnson & Johnson, Johnson & Johnson, Stephen Clarke Johnson & Johnson, Vani Lakshminarayanan Johnson & Johnson, Matthew J Loza Johnson & Johnson, Johnson & Johnson, Sophia Liva Johnson & Johnson, Tong Wang Johnson & Johnson, Ashley Orillion, PhD Johnson & Johnson, Johnson & Johnson, Ilia Tikhonov Johnson & Johnson, Johnson & Johnson, Erika Noss Johnson & Johnson, Johnson & Johnson.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (