

Background: Randomized clinical trials established Janus Kinase inhibitors (JAKi) as an efficacious intervention to reduce the disease activity of rheumatoid arthritis (RA), which was also associated with an improvement in fatigue [1]. Results for effectiveness of JAKi to reduce fatigue from real-world patient cohorts is limited to small studies without comparator drugs [2].
Objectives: To evaluate the effectiveness of JAKi in reducing RA-associated fatigue compared to tumor necrosis factor inhibitors (TNFi), and interleukin-6 inhibitors (IL-6i).
Methods: Utilizing data from the German biologics register RABBIT, we included patients with RA starting a new treatment with TNFi, IL-6i, or JAKi between 2017 and 2023 to allow for a minimum observation period of 6 months before database lock. Fatigue was measured using a numerical rating scale ranging from 0 to 10. Time to follow-up was modelled as a continuous covariate. For descriptive comparison we examined patient characteristics and treatment discontinuation. The course of fatigue was investigated using weighted generalized additive models to obtain estimates for non-linear curvature of fatigue over a 6-month follow-up period. Differences between treatments were estimated using contrasts, i.e. mean differences in fatigue with 95% confidence intervals (CI). To account for missing data, 20 multiple imputations were applied and balance between treatments was obtained using entropy based weighting [3]. In sensitivity analysis, the study population was restricted to patients with no concomitant use of methotrexate (MTX) which was reported to induce fatigue [4].
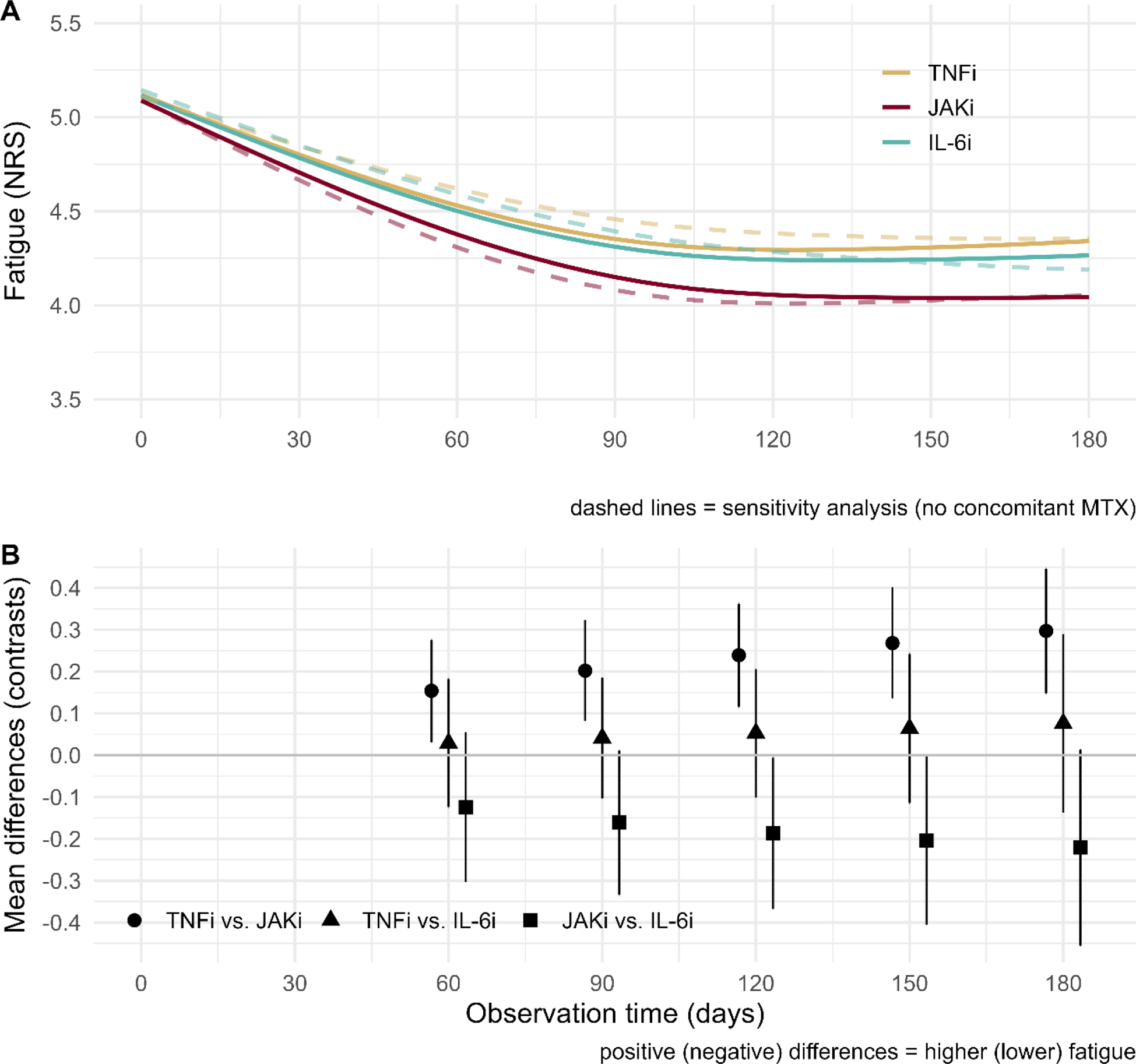
Results: In the study period, 1224 patients started therapy with JAKi, 2839 with TNFi, and 478 with IL-6i. Patient characteristics showed only minor differences between treatments in terms of age (TNFi: 59.1, JAKi: 59.5, IL-6i: 59.0 years), distribution of sex (females: 74.7%, 74.3%, and 70.4%), disease activity (DAS28-ESR: 4.53, 4.65, 4.75), and fatigue (NRS: 5.00, 5.26, 5.25). Similarly, discontinuation within 6 months was comparable between therapies (TNFi: 9.33%, JAKi: 9.0%, IL-6i: 10.9%). After weighing the study sample, the treatment groups were exactly balanced. However, larger differences were found for prior DMARD failures and the concomitant use of MTX (TNFi: 55.9%, JAKi: 37.3%, IL-6i: 31.8%) that could not be eliminated by weighting. Patients with no concomitant use of MTX were more often female, had higher pain and fatigue scores, and had more often psychological disorders. After balancing, from uniform 5.1 (95% CI) [5.0; 5.2] at baseline in all groups, treatment with TNFi reduced fatigue to 4.4 [4.3; 4.4] on day 90, and 4.3 [4.2; 4.4] on day 180 of follow-up. Treatment with IL-6i reduced fatigue to 4.3 [4.2; 4.5] and 4.3 [4.1; 4.5] respectively. Largest and earliest reduction of fatigue was found for JAKi with 4.2 [4.0; 4.3] on day 90 and 4.0 [3.9; 4.2] at day 180 (Figure 1, panel A). Differences in means between treatments (Figure 1, panel B) obtained statistical significance but did not reach minimally important differences. Similar patterns of improvement were found in sensitivity analysis of patients with no concomitant use of MTX (Figure 1, panel A, dashed lines).
Conclusion: In this large real-world patient cohort, all targeted therapies reduced fatigue considerably. Treatment with JAKi resulted in a more rapid and more pronounced reduction in fatigue in comparison to TNFi.
REFERENCES: [1] Tóth L, Juhász MF, Szabó L, Abada A, Kiss F, Hegyi P, et al. Janus Kinase Inhibitors Improve Disease Activity and Patient-Reported Outcomes in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of 24,135 Patients. Int J Mol Sci. 2022;23(3).
[2] La Barbera L, Rizzo C, Camarda F, Atzeni F, Miceli G, Molica Colella AB, et al. Effectiveness and safety of filgotinib in rheumatoid arthritis: a real-life multicentre experience. Clin Exp Rheumatol. 2024;42(5):991-8.
[3] Hainmueller J. Entropy Balancing for Causal Effects: A Multivariate Reweighting Method to Produce Balanced Samples in Observational Studies. Political Analysis. 2012;20(1):25-46.
[4] Pope JE. Management of Fatigue in Rheumatoid Arthritis. RMD Open. 2020;6(1):e001084.
Funding: RABBIT is supported by a joint, unconditional grant from AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Galapagos, Hexal, Eli Lilly and Company, MSD, Pfizer, Samsung Biosepis, Sanofi Aventis, Viatris Sante, and UCB, and previously by Roche.
Acknowledgements: NIL.
Disclosure of Interests: Adrian Richter: None declared, Martin Schaefer: None declared, Matthias Worsch: None declared, Thilo Klopsch: None declared, Matthias Schneider: None declared, Anja Strangfeld AbbVie, AlfaSigma/Galapagos, Janssen, Lilly, Pfizer, Takeda, UCB, Unconditional grant paid to my institution for the RABBIT register with equal parts from AbbVie, Amgen, BMS, Celltrion, Fresenius Kabi, Galapagos, Hexal, Lilly, MSD, Viatris, Pfizer, Roche, Samsung Bioepis, Sanofi-Aventis, and UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (