

Background: tsDMARDs represent a new important class in the armamentarium of Rheumatoid Arthritis (RA) treatments. It is unknown which specific bDMARD or mode of action should be selected after stopping a JAKi.
Objectives: Our aim was to examine if clinical response differs between advanced therapies that are initiated after stopping a JAKi.
Methods: Patients were included from the electronic platform “Tool for Administrative Reimbursement Drug Information Sharing” (TARDIS). Data from all Belgian RA patients on biologic and targeted therapy are collected via this platform during the submission of a request for initiation and prolongation of reimbursement of these drugs. Patients were selected for this analysis if they had stopped JAKi therapy and initiated a subsequent therapy. Patients were grouped by TNFi, T/B cell therapy, IL6 inhibition or JAKi therapy. The DAS28 response and proportion of patients in remission at the first 3 follow-up (after 3 to 6 months, after 15 to 18 months, and 27 to 30 months) moments were compared between groups. Remission was defined as DAS28<2.6. Sensitivity analyses took treatment line into account. Mixed-effects models were applied to assess the association between time, treatment groups, and their interaction on clinical outcomes, with random intercepts and slopes included to account for patient-level variability and repeated measures over time.
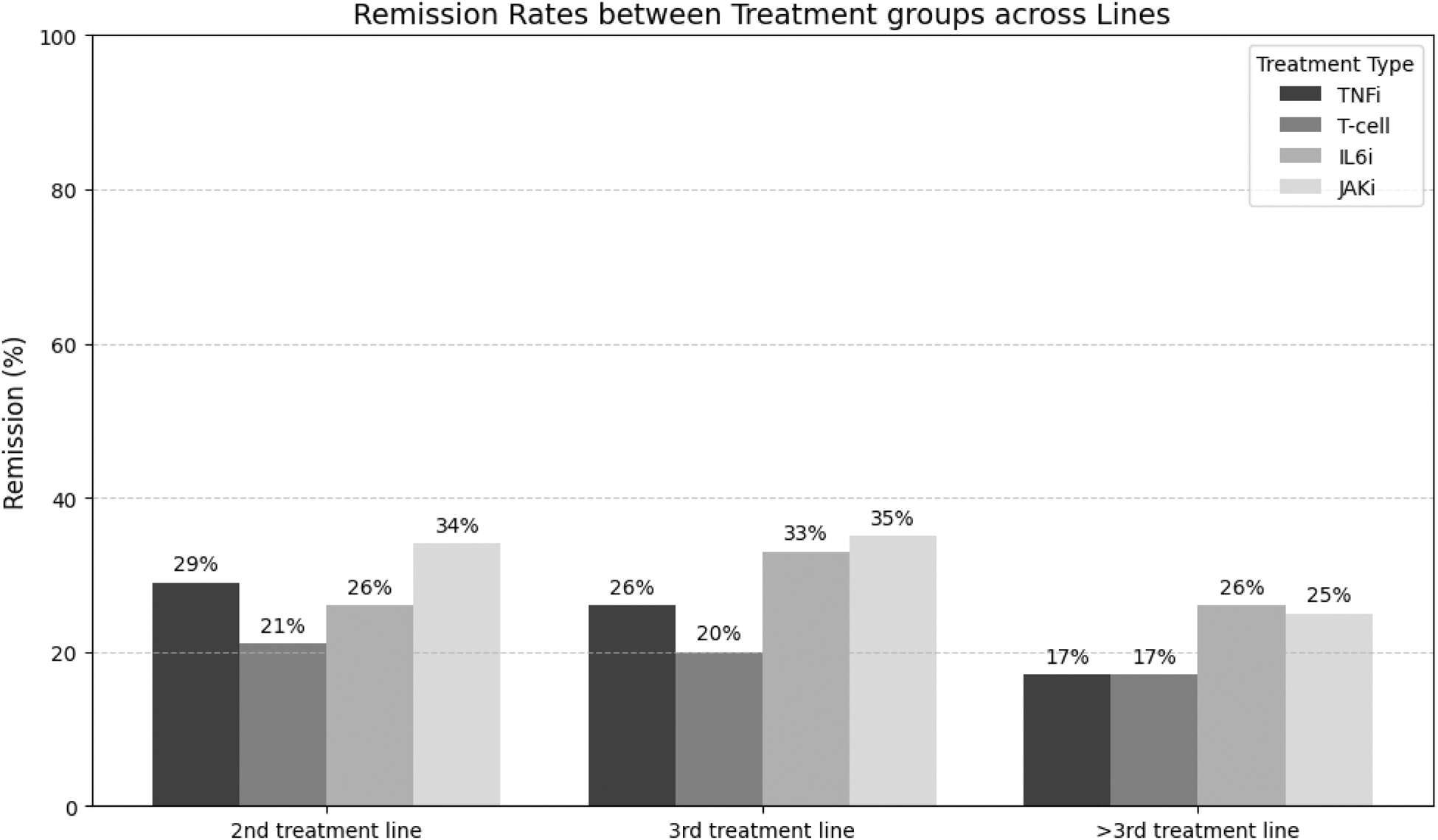
Results: In total, 2389 RA patients who had stopped JAKi therapy could be included. TNFi, T/B cell therapy, IL6 inhibition or JAKi therapy was initiated in 34% (821/2389), 18% (437/2389), 18% (423/2389) and 30% (708/2389) of these patients respectively. Baseline demographic and clinical characteristics differed between groups in most variables (Table 1). Of these 2389 patients, 1274 (53%%) patients had follow-up clinical data. Subsequently, patients on rituximab were excluded as these were retreated and their data collected only in case of flare, following Belgian reimbursement criteria. Hence, the B/T cell group was now reduced to only Abatacept. Moreover, more patients on tsDMARD therapy (12%) were in remission at baseline compared to the other bDMARD groups (5%-6%). Hence, we choose to omit patients in remission at baseline to exclude non-medical switches. Finally, 1190 (50%) patients could be included for analysis. Figure 1 show remission rates per follow-up moment between groups. At the 1 st follow-up, Abatacept had lower remission rates compared to the other therapies (p=0.008). This difference disappeared at the two other follow-up moments (p=0.268, p=0.135), probably due to a healthy survivor effect. Figure 2 details the effect of treatment line on remission rates between groups. Abatacept scored in general lower compared to the other groups in each treatment line category. An unadjusted mixed-effects model revealed that Abatacept had a lower DAS28 response (-0.151 95%CI(-0.280 to -0.022) compared to the tsDMARD reference group (p=0.021). An adjusted mixed-effects model adding baseline disease duration, ESR, CRP, PGA, SJC28 and TJC28 as random effects and treatment line as fixed effect showed again that Abatacept had a lower DAS28 response (-0.299 95%CI (-0.499 to -0.003) compared to the tsDMARD reference group (p=0.021).
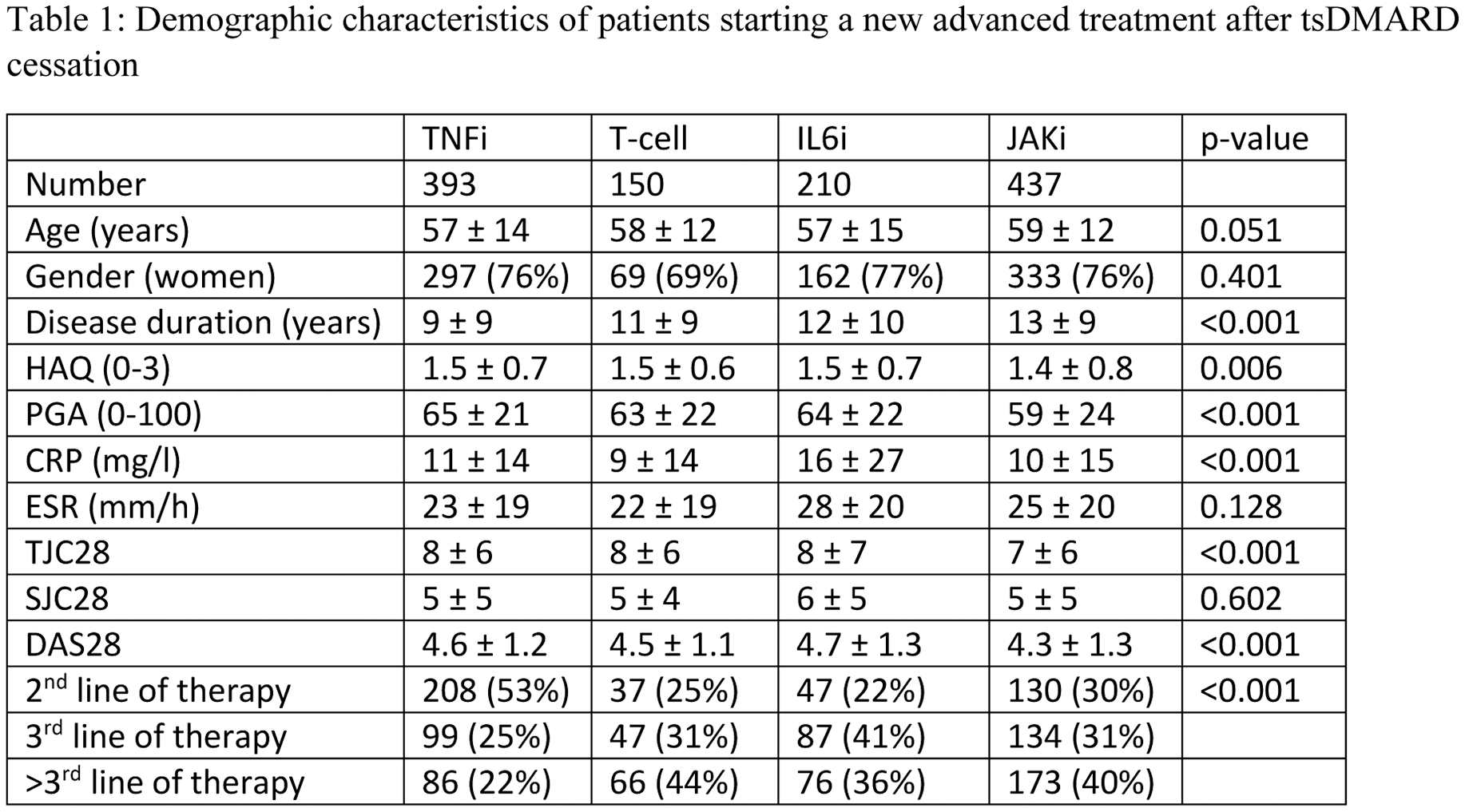
Number given are mean ± SD or number, proportion. TNFi = tumour necrosis factor inhibitor, ts= targeted synthetic, HAQ= health assessment questionnaire, PGA= Patient Global assessment; CRP= C-reactive protein; ESR= erythrocyte sedimentation rate; TJC= tender joint count; SJC= Swollen joint Count; DAS28 = disease activity score based on the 28joints
Remission rates per group per follow-up moment.
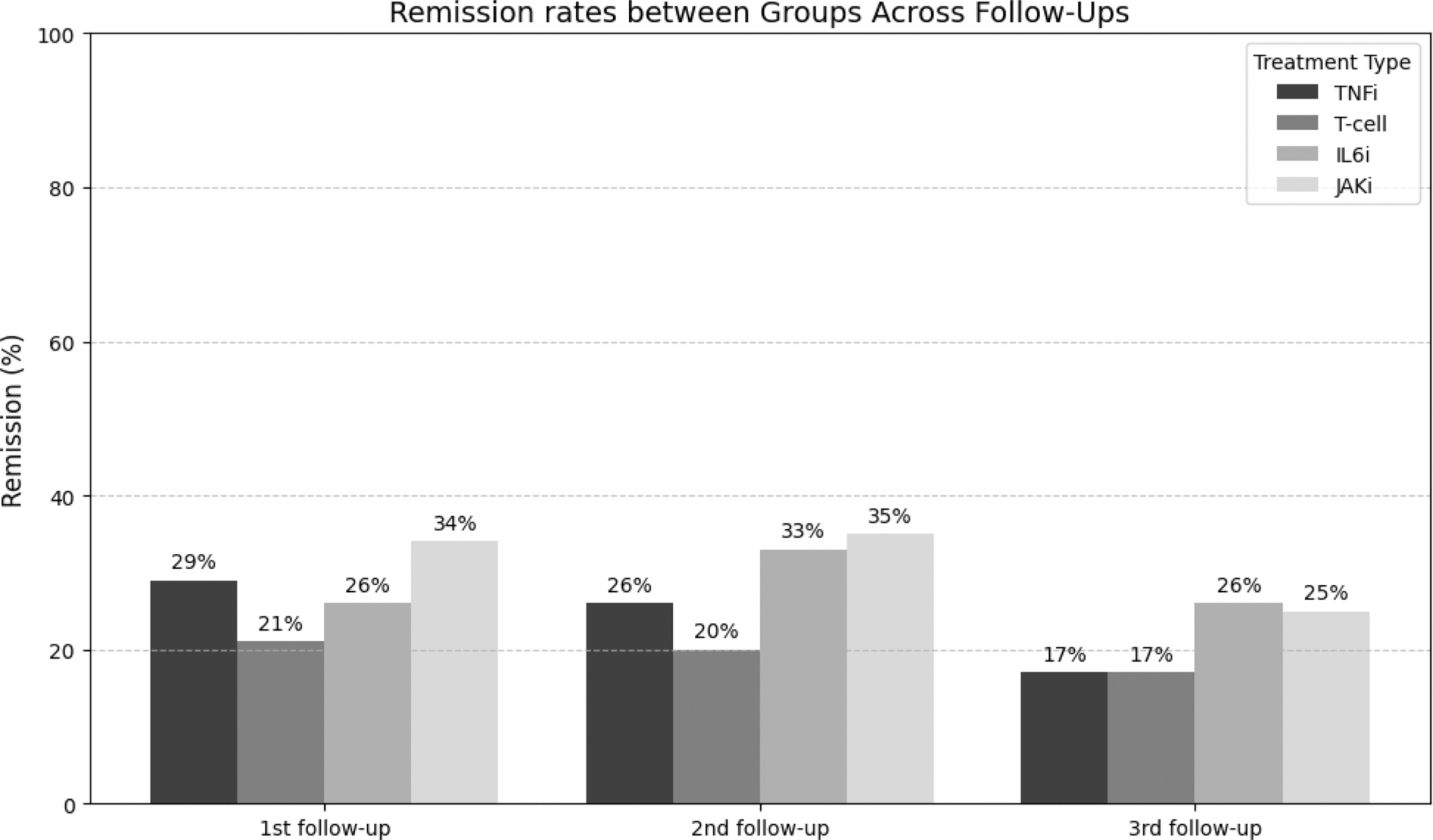
Remission rates per group per follow-up moment.
Conclusion: This extensive real-world study highlights that rheumatologists have multiple effective options following discontinuation of a first JAKi. Both TNFi and IL6i switchers, as well as JAKi cyclers, demonstrated satisfactory outcomes. However, abatacept appears to be a less favourable choice in many cases. A key strength of this analysis is the effort to minimize non-medical switches (e.g., switching between JAK inhibitors without clear clinical justification). Nonetheless, a comprehensive evaluation of prior exposure to advanced therapies is necessary to fully address our hypothesis.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (