

Background: Biologic disease-modifying antirheumatic drugs (bDMARDs) have revolutionized the treatment of juvenile idiopathic arthritis (JIA), providing targeted therapies for children with moderate to severe disease. This study investigates the evolving role of biologics and Janus kinase inhibitors (JAKi) in pediatric rheumatology over the past 25 years in Germany using data from the BIKER-Registry [1].
Objectives: This study aims to analyze trends in the utilization of bDMARDs and JAKi in JIA in Germany (2000–2024), comparing usage across JIA categories, including polyarticular JIA (pJIA), systemic JIA (sJIA), enthesitis-related arthritis (ERA), juvenile psoriatic arthritis (jPsA), and persistent oligoarthritis (pOJIA) in children.
Methods: We conducted a retrospective cohort analysis using data from the BIKER-Registry (2000–2024), focusing on the use of bDMARDs and JAK Inhibitors across different JIA categories. The analysis covered 6,263 treatment initiations among 4,195 patients. Temporal trends in therapy utilization were assessed with descriptive statistics and graphical representations.
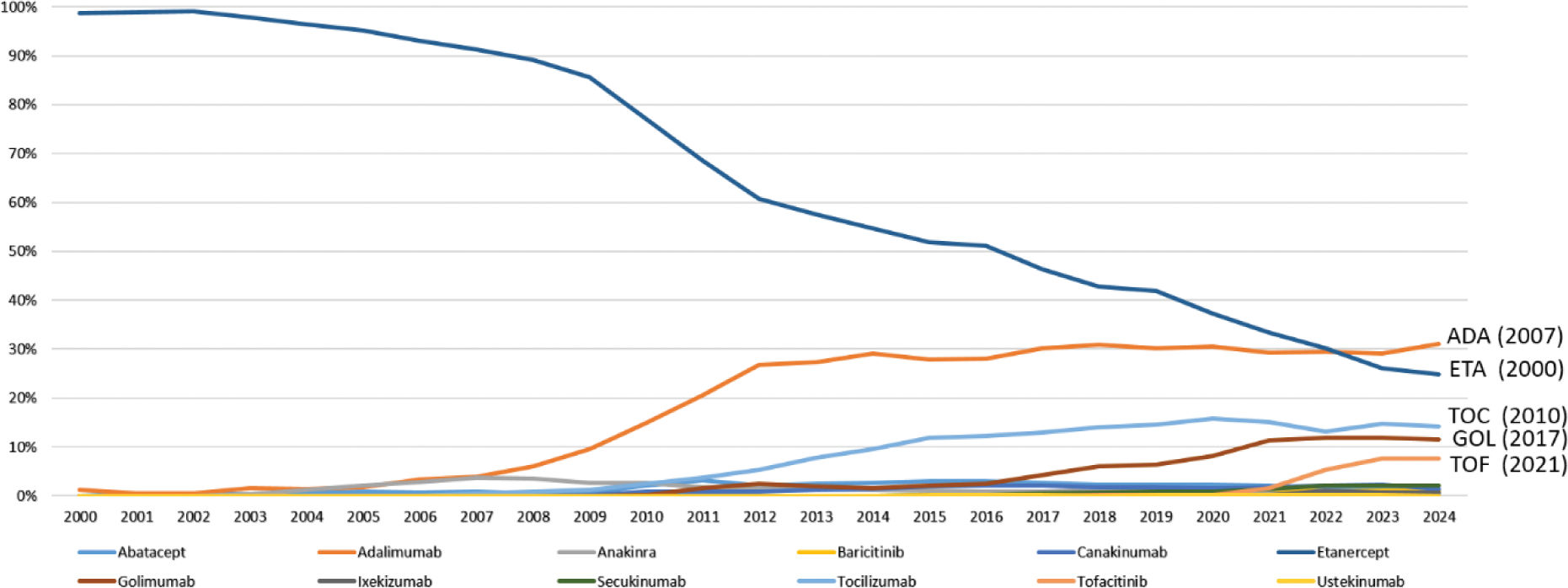
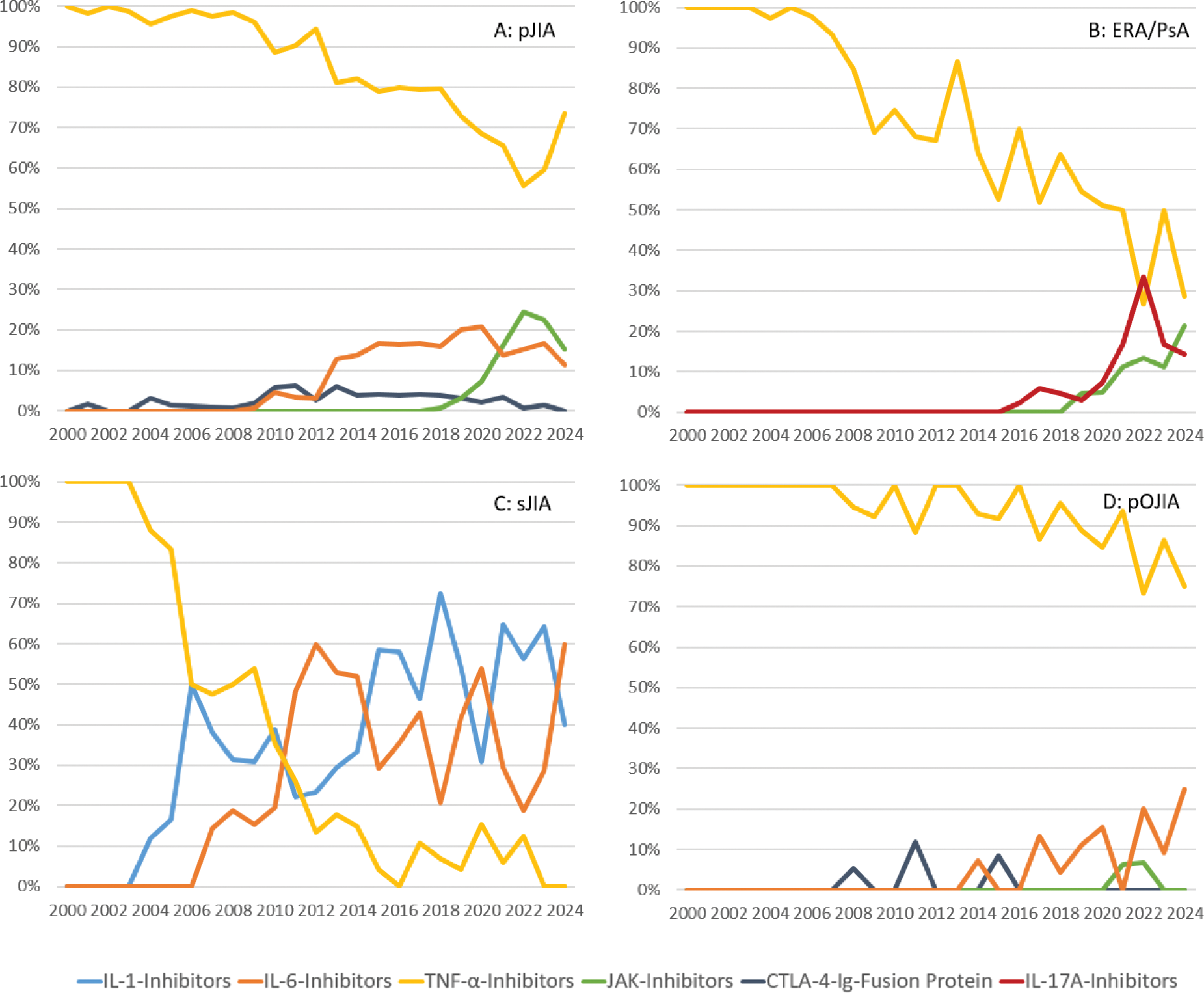
Results: Etanercept dominated the bDMARD usage for many years, accounting for over 90% of therapies until 2007. It was surpassed by Adalimumab in 2023, which accounted for 29% of usage compared to 26.1% for Etanercept (Figure 1). Since Adalimumab belongs to the TNF inhibitors (TNFi) as Etanercept, this shift reflects a change in the specific medications used rather than a departure from TNFi as the dominant treatment class. Over the past five years, TNFi still accounted for an average of 72.4% of the total bDMARD usage. Newer treatment options, such as IL-6 inhibitors (16.6%) and JAK inhibitors (6.1%), emerged in smaller proportions. For pJIA, TNFi remained the primary treatment. Abatacept, as a CTLA-4 fusion protein, accounted for a small proportion of initiations and JAK inhibitors peaked at 24% of initiations in 2022 (Figure 2A). TNFi transitioned from being the sole treatment option for sJIA to no new initiations. While until 2009, TNFi still accounted for 54% of new starts, usage steadily declined as IL-1 and IL-6 inhibitors became the preferred therapies, alternating in utilization over time (Figure 2C). In ERA/PsA, TNFi continued to dominate, while JAK inhibitors and IL-17 inhibitors collectively accounted for an average of 23.2% of new treatments (Figure 2B). For pOJIA TNFi maintained a dominant share of treatment initiation. Abatacept had a recurring impact on treatment patterns but is rarely used overall. Over recent years, IL-6 inhibitors have gained increasing significance, now accounting for approximately 25% of new treatments. (Figure 2D).
Conclusion: Over the past 25 years, TNF-alpha inhibitors have remained the cornerstone of JIA treatment, with newer biologics like IL-6 inhibitors, JAK inhibitors, and IL-17A inhibitors expanding treatment options, particularly for complex JIA categories.
REFERENCES: [1] Horneff G, Schulz AC, Klotsche J, Hospach A, Minden K, Foeldvari I, Trauzeddel R, Ganser G, Weller-Heinemann F, Haas JP. Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry. Arthritis Res Ther. 2017 Nov 22;19(1):256. doi: 10.1186/s13075-017-1462-2. PMID: 29166924; PMCID: PMC5700562.
Percentage of JIA Patients receiving Biologic DMARDs and JAK-Inhibitors in the respective year and dates of first approval in Europe (2000–2024)
Initiations of Biologic DMARDs and JAK-Inhibitors in Patients with polyarticular JIA (pJIA), systemic JIA (sJIA), enthesitis-related arthritis (ERA), juvenile psoriatic arthritis (jPsA), and persistent oligoarthritis (pOJIA) (in %).
Acknowledgements: NIL.
Disclosure of Interests: Clara Hillekamp: None declared, Angela Zimmer Speakers bureau: Lilly, Glaxo-Smith-Kline, Travel grands: Novartis, Ariane Klein Speakers fee: Novartis, Lilly, Gerd Horneff Abbvie, Boehringer, Celgene, Chugai, GSK, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi, Sobi.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (