

Background: Familial Mediterranean Fever (FMF) is a hereditary autoinflammatory disorder characterized by recurrent fever episodes, serositis, arthritis, and erysipelas-like erythema. Mutations in the MEFV gene, particularly in exon 10, are strongly associated with FMF, contributing to its clinical heterogeneity. While colchicine remains the cornerstone treatment, its efficacy varies depending on genetic mutations. Patients with inadequate responses or intolerance often require IL-1 inhibitors, such as anakinra or canakinumab, which are effective but costly. Recent advances in next-generation sequencing (NGS) have highlighted the presence of other autoinflammatory syndromes in FMF patients, particularly in those with atypical presentations or MEFV-negative status and most of them are more responsive to IL-1 inhibitors rather than colchicine. Understanding the impact of MEFV mutations on treatment efficacy, safety, and clinical outcomes is critical to optimizing patient management.
Objectives: This study aimed to assess the influence of MEFV mutations on colchicine efficacy and safety, as well as the need for IL-1 inhibitors in FMF patients. Additionally, it explored clinical and laboratory changes after colchicine treatment, comparing MEFV-positive and MEFV-negative patients. The goal was to identify diagnostic and therapeutic challenges, particularly in atypical cases, and provide insights into improving personalized treatment strategies.
Methods: A retrospective cohort study was conducted on FMF patients between April 2020 and June 2024. Patients were classified into MEFV-positive and MEFV-negative groups based on genetic testing results. MEFV-positive patients included those with exon 10 mutations (homozygous or heterozygous), while MEFV-negative patients had no pathogenic mutations or only benign mutations (e.g., R202Q, E148Q). Data collected included demographics, clinical features, age at symptom onset, diagnostic delays, treatment initiation timelines, given treatment agents and adverse effect profiles. Additionally, laboratory findings, attack characteristics, treatment responses to colchicine, and the transition to IL-1 inhibitors were analyzed. Data were gathered through direct interviews and patient records.
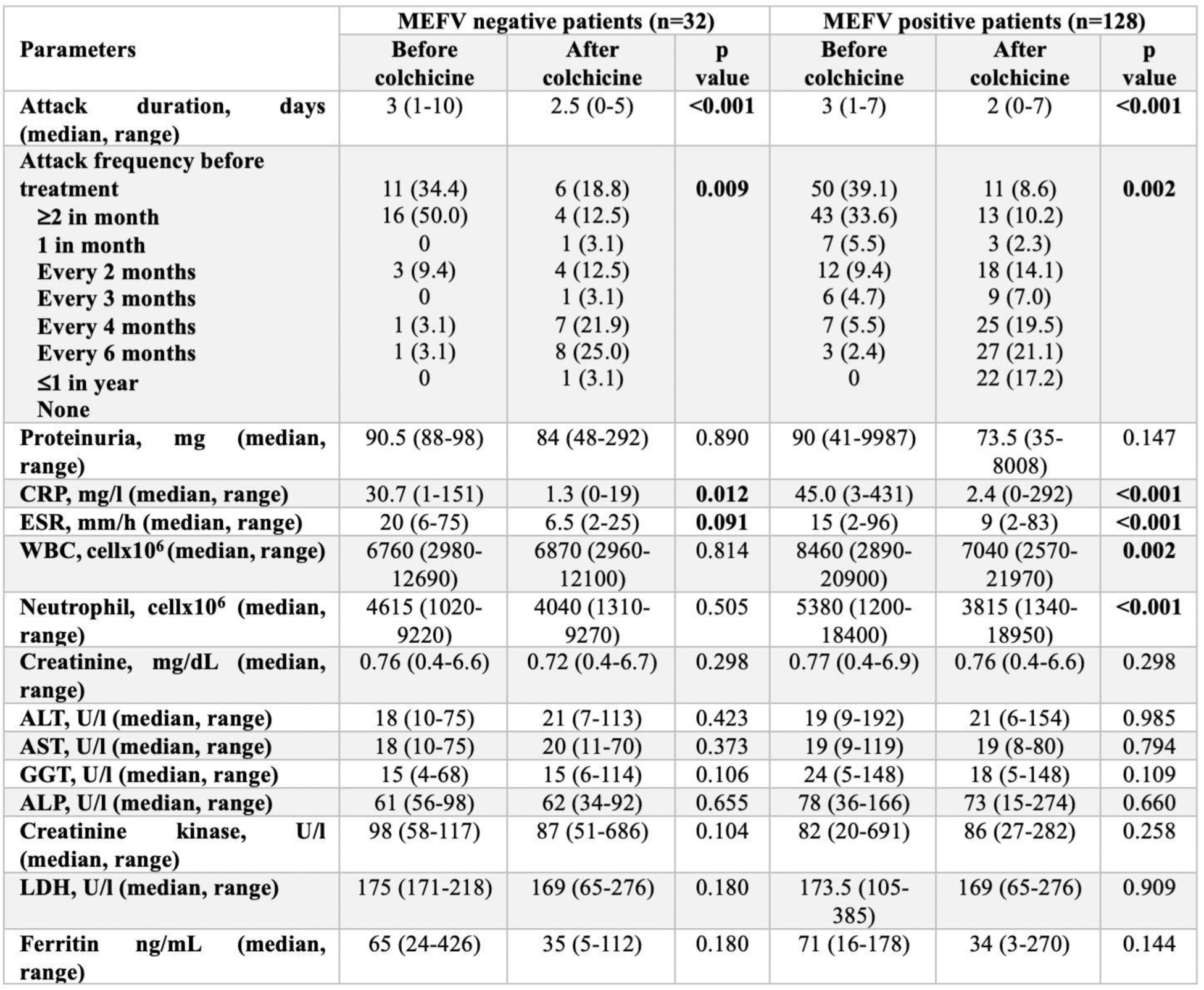
Results: The study included 160 FMF patients, comprising 128 MEFV-positive and 32 MEFV-negative cases. Demographic and clinical features revealed a longer median duration between symptom onset and diagnosis in MEFV-negative patients (10.5 vs. 5 years, p=0.075) and between symptom onset and treatment initiation (10 vs. 5 years, p=0.131). Diagnostic processes and treatment detailes were given in Table 1. Before colchicine treatment both group higher attack frequencies. After treatment, attack frequency significantly decreased in both groups, with MEFV-positive patients exhibiting a higher percentage of complete remission. Attack duration before treatment was comparable between the groups and improved in both groups after treatment. Table 2 highlights significant post-treatment improvements, with MEFV-positive patients showing better inflammation control. Both groups exhibited significant improvements in attack characteristics after colchicine treatment. However, MEFV-positive patients experienced more substantial reductions in acute-phase reactants, with C-reactive protein and erythrocyte sedimentation rate levels showing greater declines compared to MEFV-negative patients. All patients received colchicine but although not significant, MEFV-negative patients exhibited more adverse effects and distribution of advers effects were heterogenous among study groups, reflecting differences in colchicine metabolism among them.
Conclusion: The study highlights significant clinical and therapeutic differences between MEFV-positive and MEFV-negative FMF patients. MEFV-negative patients present diagnostic challenges due to atypical features and prolonged diagnostic timelines, often requiring more advanced genetic testing and tailored treatment strategies. While colchicine effectively reduces attack severity and inflammation in both groups, MEFV-positive patients demonstrated superior inflammation control. The differences in adverse event profiles and the more effective inflammation control observed in MEFV-positive patients support the potential of colchicine to regulate pyrin-mediated inflammation, particularly in typical FMF cases with exon 10 mutations. Implementing NGS-based exome sequencing and broader autoinflammatory panels can refine diagnoses, particularly in MEFV-negative or colchicine-refractory cases. This approach enables more precise, cost-effective management and improved patient outcomes, supporting targeted therapies for overlapping or isolated autoinflammatory syndromes. Future research should focus on integrating genetic findings with clinical phenotypes to develop personalized treatment strategies.
REFERENCES: NIL.
Table 1. Baseline demographics, diagnosis process and treatment history in patients according to MEFV positivity
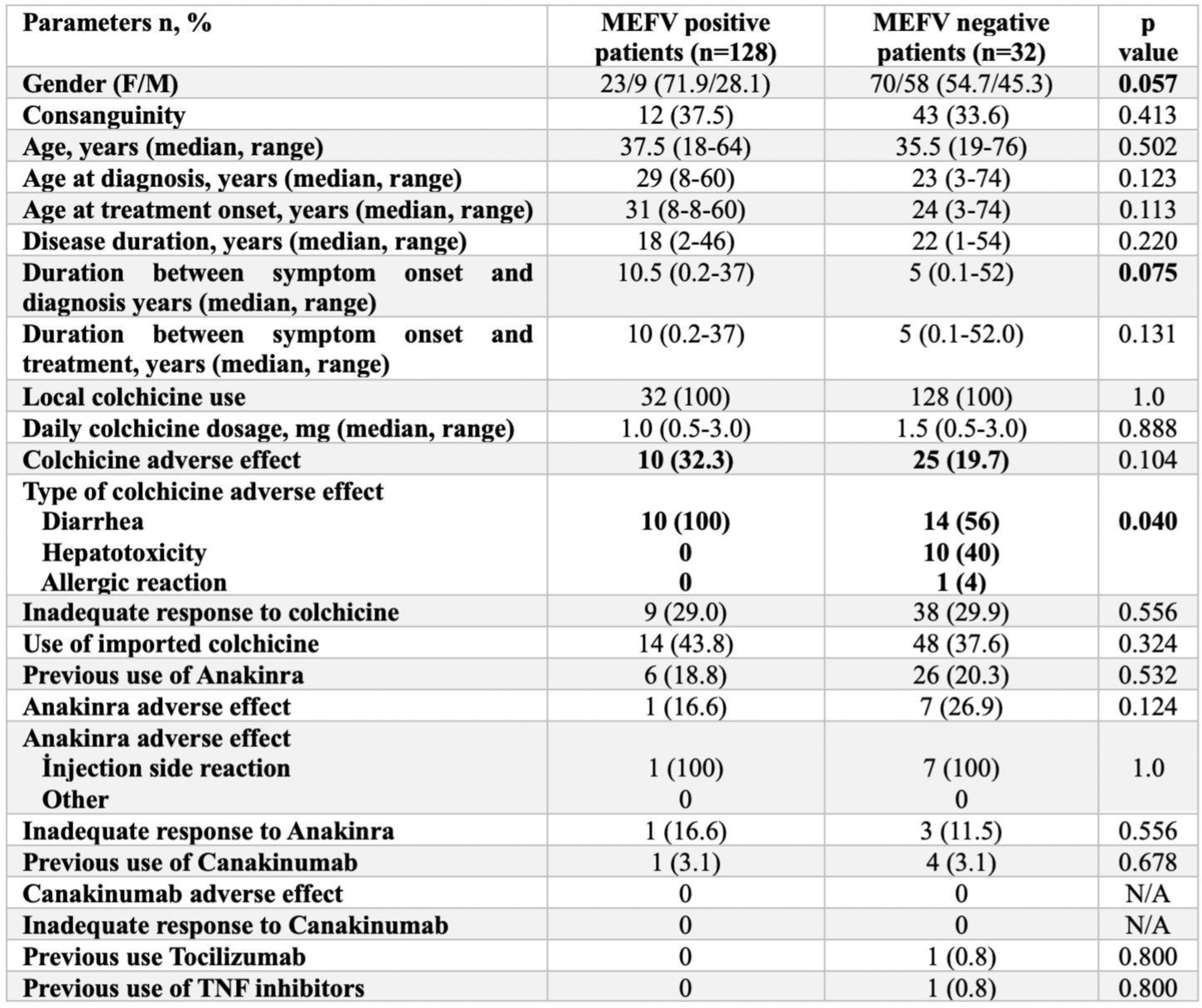
Table 2. Changes in attack characteristics and laboratory parameters after colchicine treatment according to MEFV positivity
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (