

Background: The majority of currently available data on patients with familial Mediterranean fever (FMF) are retrospective and based on single-center, national experiences.
Objectives: We present in detail the real-life data from the FMF cohort of the longitudinal, international registry of EuroFever.
Methods: The INSAID/Infevers classification to MEFV genetic variants were applied to the entire cohort [1]. Patients fulfilling the current genetic and clinical Eurofever/PRINTO classification criteria (a suggestive clinical phenotype and carrying at least one pathogenic MEFV variant of two VUS) were considered as FMF+, while patients only diagnosed according to the clinical manifestations were considered as FMF- and analyzed as control group [2].
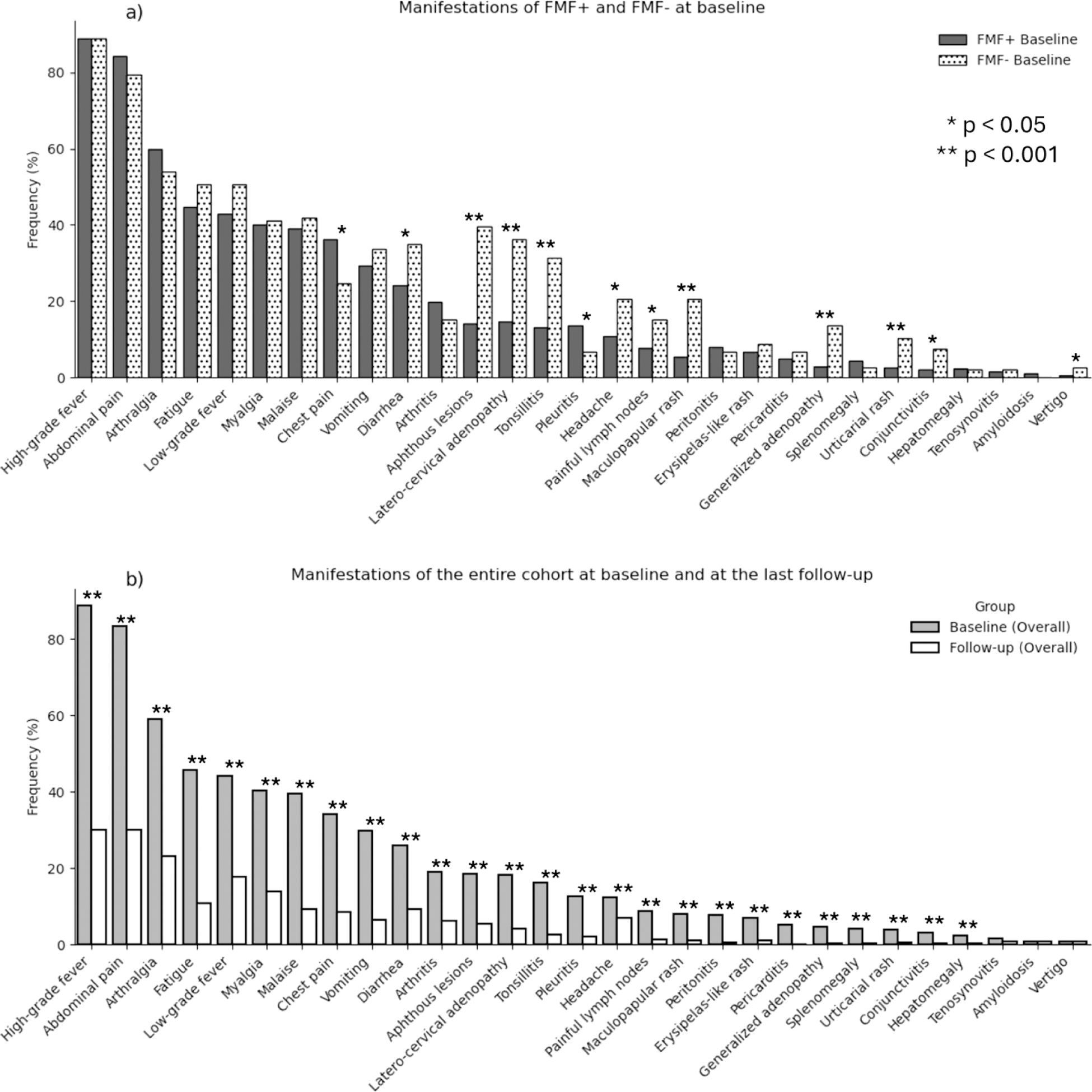
Results: In November 2024, 876 patients (466 M, 410 F) were enrolled. The median age at onset was 3.57 years (0 - 59.08), with a mean follow-up time of 2.9 ± 3.1 years. 349 (39.8%) patients carried a confirmatory MEFV genotype, with M694V being the prevalent variant (484, 55.3%). 730 (84%) patients were positive to FMF genetic and clinical criteria (FMF+), 146 (16%) did not display an informative MEFV genotype (FMF-). At baseline, the most frequent symptoms were fever (778, 88.8%) and abdominal pain (731, 83.4%), with a global reduction of almost all the manifestations during the follow-up (Figure 1). FMF+ patients had shorter episode durations at baseline (3[2-3] vs 3 [3-5] days) and at the last follow-up - (2[1-3] vs 3 [2-3] days) than FMF-. At baseline, FMF+ patients had higher rates of chest pain (36.2% vs 24.7%, p=0.01) and pleuritis (13.7% vs 6.8%, p=0.032), while FMF− patients showed more aphthous lesions (39.7% vs 14.2%, p<0.001), latero-cervical adenopathy (36.3% vs 14.8%, p<0.001), and tonsillitis (31.5% vs 13.2%, p<0.001). At the last follow-up, the number of patients treated with colchicine (749, 85.5%), was similar to the baseline (724, 82.6%), without significant variations of dosage (median 1 [1-1.5] mg/day) between groups with different disease activity. Anti IL-1 treatment was ongoing in 133 patients (15.2%), mostly canakinumab (117, 13.4%), with a median dosage of 150 [73.3-150] mg/4 weeks (Table 1). An optimal compliance (defined as taking > 90% of prescribed doses) was observed in 72.5% of visits for patients on colchicine, 48.2% for those on anakinra, and 80.7% for patients on canakinumab. At the last visit, 433 (50.6%) patients still had some disease activity, with FMF- patients showing a risk 1.6 greater than FMF + patients of having an incomplete clinical response rather than a complete clinical remission. 162 adverse events were reported during the whole observational study, mostly upper respiratory tract infections, abdominal pain or diarrhea. 22 adverse events were surely or possibly related to the ongoing treatment (20 for colchicine, 2 for anakinra, 0 for canakinumab).
Conclusion: Patients who lack genetic confirmation display significant differences in clinical features, duration of attacks, long term-outcome and a significantly less response to colchicine. Thus, these patients should be considered as FMF-mimics and investigated for other causes. For FMF patients more than half reach complete remission with colchicine. Longitudinal data provides a detailed comprehension of the long-term burden of FMF and the impact of treatment on disease activity and patients’ quality of life.
REFERENCES: [1] Van Gijn ME, et al. New workflow for classification of genetic variants’ pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID). J Med Genet. 2018;55(8):530-537.
[2] Gattorno M et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis. 2019;78(8):1025-1032.
Treatment in longitudinal cohort: baseline and at the last follow-up. FMF+: patients fulfilling genetic and clinical FMF criteria, FMF-: patients not fulfilling the genetic criteria for FMF [2].
| Treatment | At baseline | At last follow-up |
|---|---|---|
|
Off therapy, n (%
)
| 125 (14,3%)
| 97 (11,1%)
|
|
Colchicine, n (%
)
| 724 (82.64%)
| 749 (85.5%)
|
| Duration in years, median [Q1-Q3] | 1.82 [0.23 – 5.17] | 4.19 [1.66 – 7.9] |
| Dosage in mg/day, median [Q1-Q3] | 1.00 [0.75 – 1.5] | 1.00 [1.0 – 1.5] |
| Dosage in mg per kg/day, median [Q1-Q3] | 0.03 [0.02 - 0.04] | 0.03 [0.02 - 0.03] |
|
Anakinra, n (%
)
| 18 (2.1%)
| 16 (1.8%)
|
| Duration in years, median [Q1-Q3] | 1.10 [0.41- 3.31] | 1.06 [0.47– 2.91] |
| Dosage in mg/day, median [Q1-Q3] | 100 [75.0 – 100] | 100 [93.75-100] |
| Dosage in mg per kg/day, median [Q1-Q3] | 1.85 [1.4 – 2.2] | 1.75 [1.39 – 2.1] |
|
Canakinumab, n (%
)
| 59 (6.7%)
| 117 (13.4%)
|
| Duration in years, median [Q1-Q3] | 1.66 [0.58- 3.63] | 1.53 [0.48 – 3.9] |
| Dosage in mg/4 weeks, median [Q1-Q3] | 150 [66.7-150] | 150 [73.3 – 150.0] |
| Dosage in mg per kg/4 weeks, median [Q1-Q3] | 2.31 [1.45 – 3.33] | 2.32 [1.69 – 3.13] |
Clinical manifestations depending on the follow-up and the genotype. a) manifestations at baseline in FMF+ and FMF- patients; b) manifestations of the entire cohort at baseline and at the last follow-up visit.
Acknowledgements: The Authors would like to thank Eugenia Mosci and Elisa Patrone for their commitment in the secretarial assistance for this study. The project has been funded by E-Rare-3 project (INSAID, grant 003037603). Eurofever was supported by the Executive Agency For Health and Consumers (EAHC, Project No 2007332). Novartis provided an unrestricted financial support to the study.
Disclosure of Interests: Saverio La Bella: None declared, Marta Bustaffa: None declared, Yagmur Bayindir: None declared, Gayane Amaryan: None declared, Romina Gallizzi: None declared, Efimia Papadopoulou-Alataki: None declared, Giovanna Fabio: None declared, Naiera Assalia: None declared, Gil Amarilyo: None declared, Milos Jesenak: None declared, Luciana Breda: None declared, Jordi Anton Novartis, SOBI, Novartis, SOBI, Novartis, SOBI, Elizabeth Legger Novartis, SOBI, Maria Alessio: None declared, Gabriele Simonini: None declared, Donato Rigante: None declared, Laura Obici Novartis, Jasmin B. Kuemmerle-Deschner: None declared, Ozgur Kasapcopur Novartis, SOBI, Novartis, SOBI, Novartis, SOBI, Antonella Insalaco: None declared, Mia Glerup: None declared, Joost Frenkel: None declared, Juergen Brunner: None declared, Gerd Horneff: None declared, Judith Sanchez Manubens: None declared, Sevcan Bakkaloglu: None declared, Luca Cantarini: None declared, Alessandra Spagnolo: None declared, Sofia Alataki: None declared, Maria Carrabba: None declared, Annamaria Porreca: None declared, Roberta Caorsi: None declared, Nicolino Ruperto: None declared, Marco Gattorno Novartis, SOBI, Fresinius-Kabi, Novartis, SOBI, Novartis, SOBI, Seza Ozen Novartis, SOBI, Novartis, SOBI, Novartis, SOBI.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (