

Background: Vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic (VEXAS) syndrome is a monogenic autoinflammatory disorder with significant morbidity and mortality. Pathogenic variants in the UBA1 gene have been established as the key etiological factor. Common manifestations include skin lesions, fever, weight loss, lung involvement, chondritis, arthralgia, fatigue, cytopenia, myelodysplastic syndrome (MDS), thrombosis, and hematologic malignancies. Furthermore, the disease is associated with significant mortality and morbidity. Patients tend to respond most profoundly to glucocorticoids, though their use is associated with significant adverse effects. Numerous treatment options including azacitidine, JAK inhibitors, IL-6 inhibitors, anti-IL-1, and anti-TNF agents have have shown promise in alleviating symptoms and reducing glucocorticoid dependence. However, no consensus on a treatment algorithm for VEXAS syndrome has been reached.
Objectives: This study aims to evaluate the efficacy and safety of non-invasive treatment options through a meta-analysis of existing data, helping establish clearer guidelines for managing VEXAS syndrome.
Methods: The study protocol was registered in PROSPERO (CRD42024590134). MEDLINE and EMBASE were screened from inception until September 2024. We included patients with VEXAS syndrome who received treatment with azacitidine, JAK inhibitors, IL-6 inhibitors, anti-IL-1, or anti-TNF agents. The primary outcomes were the proportion of complete responders and patients who experienced adverse events. Secondary outcomes were the proportion of partial responders and the occurrence of serious adverse events. Given the lack of standardized response criteria for VEXAS syndrome, we defined complete response as clinically asymptomatic, normalization of inflammatory/hematological parameters, prednisolone <10mg/day or independence, or genetic remission (VAF <1%). Partial response was marked by symptom improvement, laboratory parameter normalization, and reduced prednisolone or VAF.
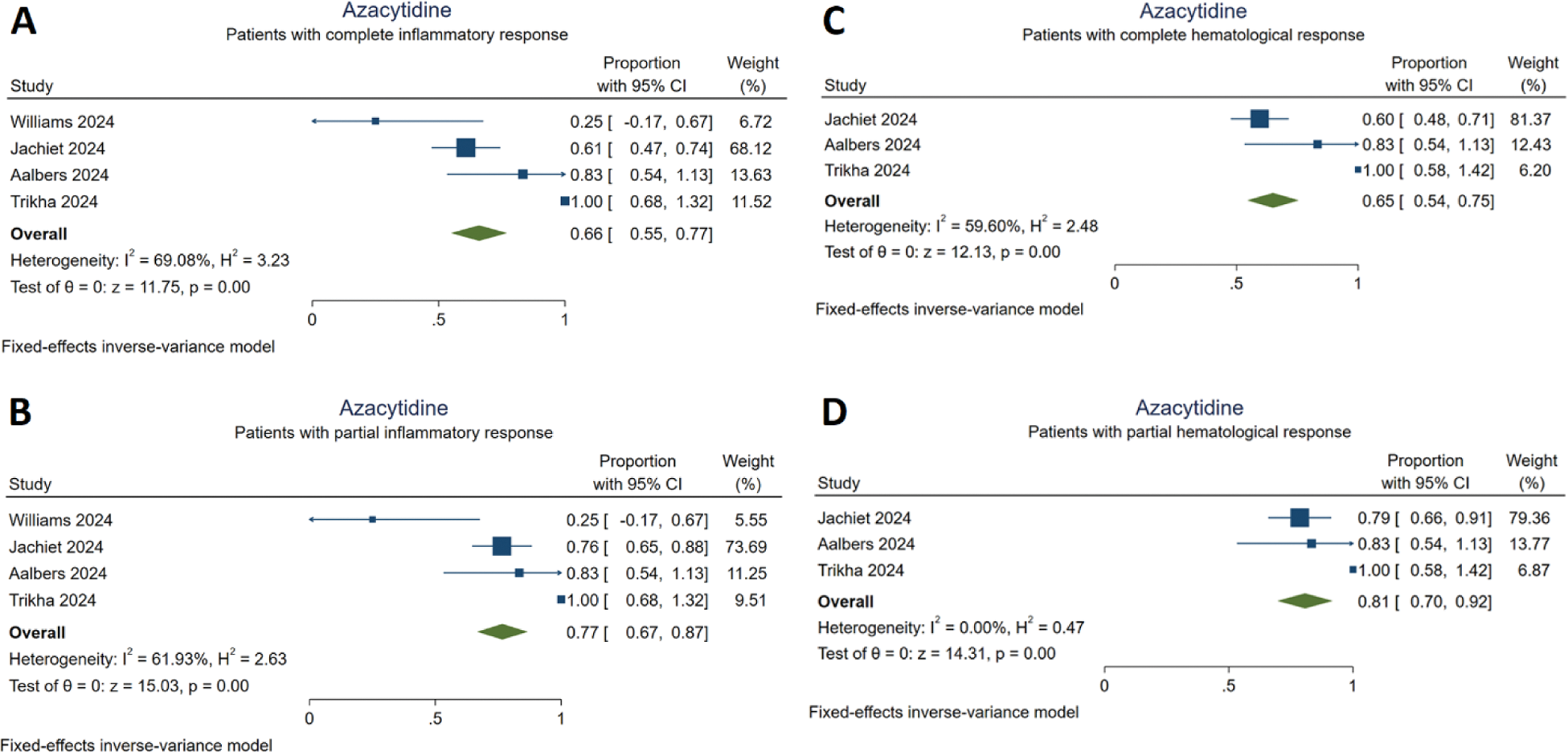
Results: A total of 15 studies and 291 patients with VEXAS syndrome were included. Detailed information about the study characteristics is presented in Table 1. Among the included patients, 116 (39.9%) were reported to have MDS. Azacitidine treatment resulted in complete and partial inflammatory response in 66% [95% CI (0.55,0.77)] and in 77% [95% CI (0.67,0.87)] of cases, respectively. Complete and partial hematological responses were achieved in 65% [95% CI (0.54,0.75)] and 81% [95% CI (0.70,0.92)] of the patients treated with azacitidine, respectively (Figure 1). Infections were frequently reported during azacitidine treatment. JAK inhibitors provided complete responses in 49% [95% CI (0.39,0.58)] and partial responses in 78% [95% CI (0.70,0.86)]. Cardiovascular adverse events were the most commonly reported serious adverse events during treatment with JAK inhibitors. IL-6 inhibitors led to a complete response in 27% [95% CI (0.18,0.36)] and partial response in 62% [95% CI (0.52,0.71)]. Anti-IL-1 treatment did not yield any significant increase in the proportion of complete responders [10%, 95% CI (0,0.21)]; however, a partial response to anti-IL-1 treatment was achieved by 35% [95% CI (0.22,0.48)] of the patients. Anti-TNF agents did not significantly increase the number of complete responders [9%, 95% CI (-0.04,0.22)] and the proportion of patients who partially responded to anti-TNF treatment was 27% [95% CI (0.09,0.45)].
Table 1. Descriptives of the included reports
Response to azacitidine treatment
A: Patients with complete inflammatory response
B: Patients with partial inflammatory response
C: Patients with complete hematological response
D: Patients with partial hematological response
Conclusion: Azacitidine and JAK inhibitors may be the treatment of choice for patients with VEXAS syndrome and concomitant MDS. IL-6 inhibitors are favorable for patients with high inflammatory activity and no hematological involvement. Anti-IL-1 and anti-TNF agents should not be considered until treatment with the aforementioned alternatives has failed. The elevated risk of infections and cardiovascular adverse events, caused by both the side effect profiles of the medications and the advanced age of the patients, necessitates close monitoring throughout treatment. Further clinical trials with a higher quality of design are needed to expand our knowledge about this topic.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (