

Background: Glucocorticoids (GCs) are important for the management of systemic lupus erythematosus (SLE); however, chronic use is associated with significant adverse effects and organ damage. Despite guidelines emphasising the importance of tapering GCs in order to minimise GC-attributable organ damage accrual, detailed recommendations on how to taper are limited.
Objectives: To develop consensus-based guidance for tapering GCs in patients with SLE, including specific tapering regimens, through the use of a modified Delphi technique.
Methods: Delphi Rounds
Following an initial literature and guideline review, candidate statements were developed and grouped into three topics:
GC-tapering regimens in lupus: general considerations
Managing flares and patients with high risk of flare during GC tapering
Managing GC-associated toxicity and GC withdrawal symptoms
To gain further insights into current practice, open-ended and multiple-choice questions were also included, and example GC-tapering regimens for different disease severities were proposed for respondents to comment on. Results were obtained over two rounds of online surveys, with Round 1 results supporting the development of Round 2. In Round 2, respondents were asked to vote for which regimens they deemed acceptable for use in clinical practice and could vote for multiple regimens per patient scenario.
Respondents
An interdisciplinary group of experts were invited to participate in two online Delphi rounds. Snowball sampling was used to identify global lupus experts including rheumatologists, nephrologists, dermatologists and internists.
Defining consensus
In Round 1, respondents were asked to rate their agreement with the candidate statements using a nine-point Likert scale, with a rating of 1–3 indicating disagreement, 4–6 indicating neutrality, and 7–9 indicating agreement. Consensus was defined as ≥70% of respondents voting either ≤3 (disagreement) or ≥7 (agreement). In Round 2, respondents were presented with the mean ratings from Round 1, their own Round 1 ratings and the opportunity to revise their rating.
Results: Of the 124 respondents invited, 101 (81%) completed Round 1 and, of these, 94 (93%) completed Round 2. Respondents who completed both rounds were from 30 countries and comprised 79 rheumatologists, 11 nephrologists, 3 dermatologists and 1 internist. Of the eight example GC-tapering regimens presented, three achieved ≥70% agreement that they would be acceptable to use in clinical practice (regimens for mild and moderate SLE and lupus nephritis). The regimens that received the highest agreement in Round 2 for each patient scenario are presented in Table 1. For patients with mild SLE, 87.2% of respondents agreed that a 4-week regimen resulting in withdrawal of GCs, if disease was controlled, was acceptable. Additionally, respondents agreed with the regimen for moderate SLE, with 93.6% voting it acceptable; however, only 59.6% of respondents considered the use of intravenous methylprednisolone appropriate for these patients. For patients with active lupus nephritis, agreement was achieved (74.5% of respondents) in using intravenous methylprednisolone at a dose of 0.25–5 g/day for up to 3 days, followed by GC tapering to <2.5 mg/day by Week 25. 88.3% of respondents agreed that this regimen could also be used for patients with severe non-renal SLE. Lastly, for patients on long-term low-dose GC treatment, 94.7% of respondents considered either the 12-week regimen, the 6-month regimen or both regimens acceptable for use (Table 1); 62.8% of respondents considered the 12-week regimen acceptable and 67.0% considered the 6-month regimen acceptable. Overall, consensus for either agreement or disagreement was achieved in 31/33 statements in Round 2 (Table 2).
Conclusion: This consensus-based guidance will support physicians in choosing a GC-tapering approach for patients with SLE across different clinical scenarios and help them to use GCs more efficiently. The next step for this guidance is to obtain the patient perspective on these results to ensure that patient preferences are considered when initiating GC tapering, followed by dissemination into routine practice.
Funding: This study was sponsored by AstraZeneca.
Table 1. Summary of recommended GC-tapering regimens
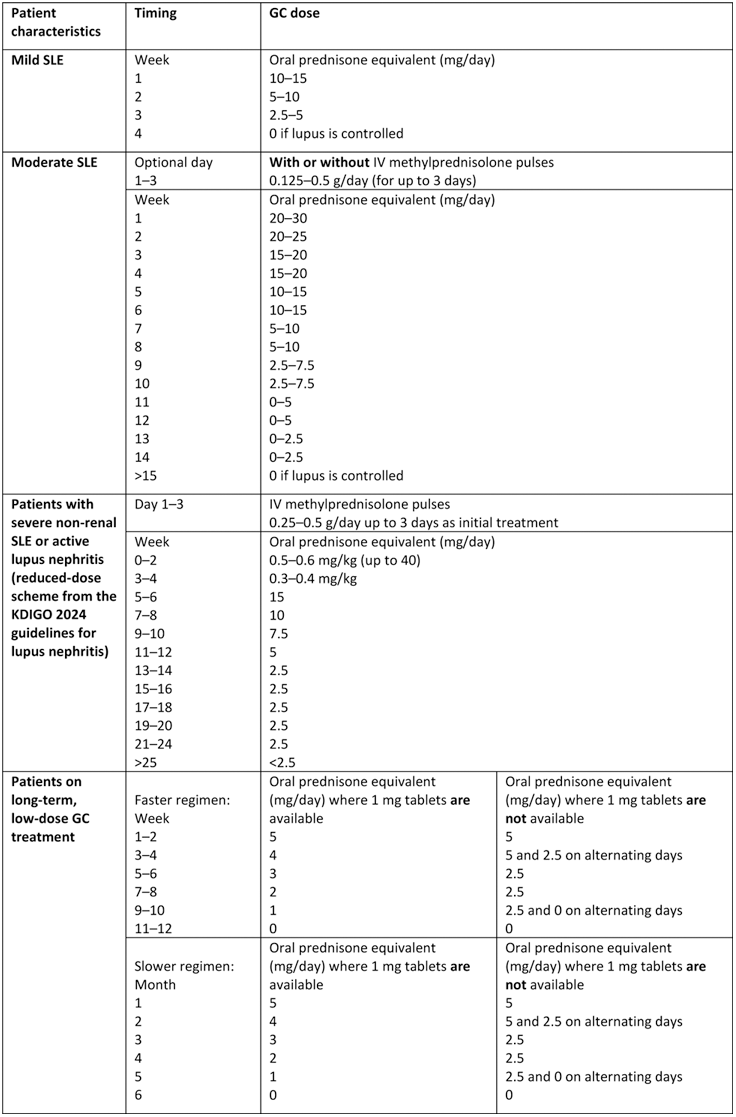
IV, intravenous; KDIGO, Kidney Disease: Improving Global Outcomes
Table 2. Consensus received on final GC-tapering voting statements
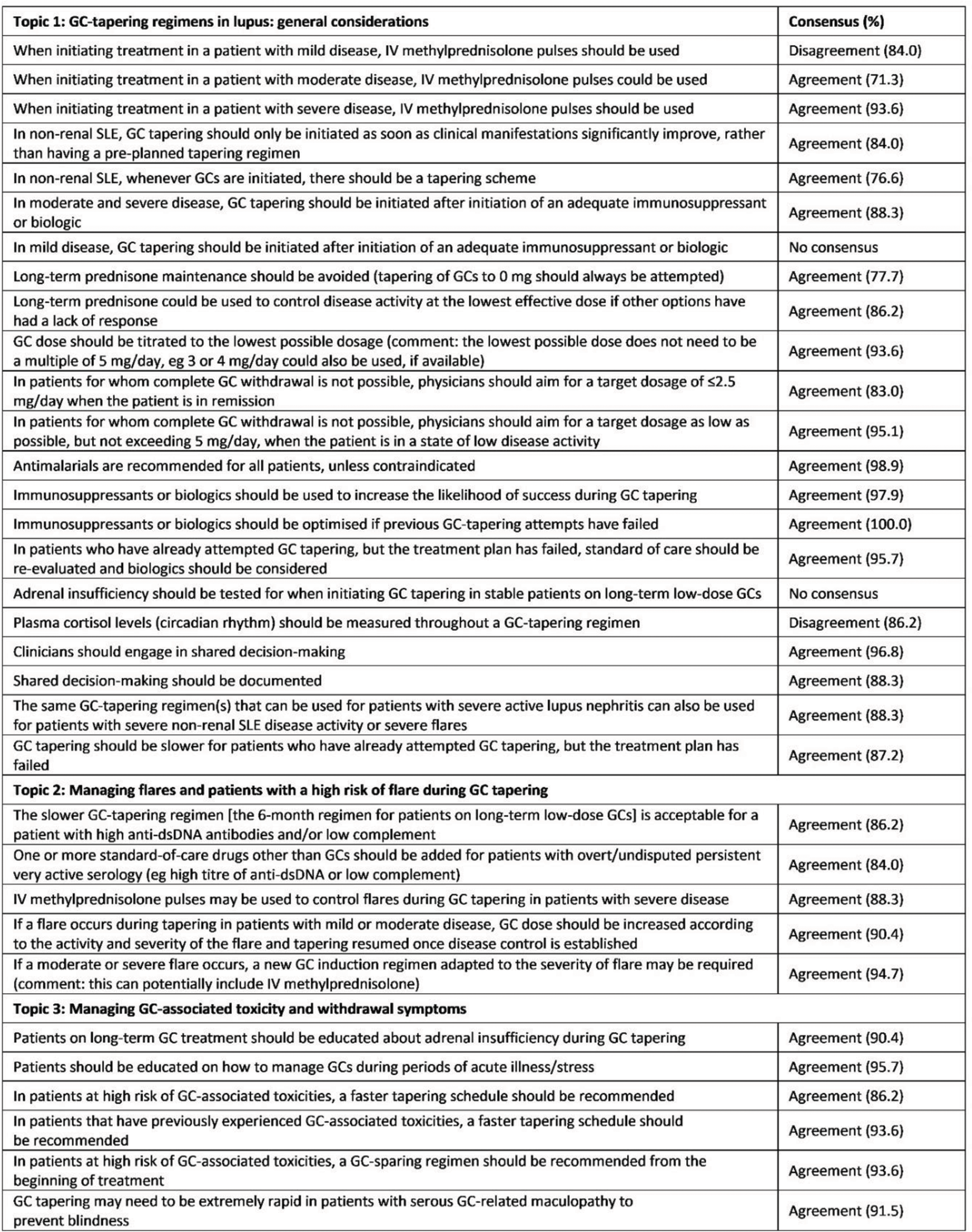
dsDNA, double-stranded DNA; IV, intravenous
REFERENCES: NIL.
Acknowledgements: Medical writing support was provided by Helios Medical Communications and was funded by AstraZeneca in accordance with Good Publication Practice (GPP) guidelines (
Disclosure of Interests: Edward M. Vital has received honoraria from Abbvie, AstraZeneca, Aurinia, BMS, Idorsia, ILTOO Pharma, Lilly, Merck, Novartis, Otsuka, Pfizer, Roche and UCB, and has received grants paid to his employer from AstraZeneca, Roche and Sandoz, George K. Bertsias has received speaker fees from AstraZeneca, GSK, Otsuka, Pfizer, Novartis and Abbvie, has received consulting fees from AstraZeneca, GSK and Novartis, and has received grants from AstraZeneca, GSK and MSD, Andrea Doria has received speaker fees from AstraZeneca, GSK and Otsuka, and has received consulting fees from AstraZeneca, Biogen, GSK, MSD and Otsuka, Sindhu R. Johnson: None declared, Sarah Mackie has received speaker fees for AbbVie, CSL Vifor, Fresenius Kabi, Novartis, Roche/Chugai Pharmaceutical and UCB, and has received consulting fees from AbbVie, AstraZeneca, Pfizer, Roche/Chugai Pharmaceutical and Sanofi, Sandra Navarra has received speaker fees from AstraZeneca and Boehringer Ingelheim, and has received consulting fees from Biogen, Bas Nijmeijer is an employee at AstraZeneca, Ayobami Olojo is a shareholder at AstraZeneca, and is an employee at AstraZeneca, Bernardo Pons-Estel has received speaker fees from AstraZeneca, GSK, Janssen and Novartis, and has received grants from AstraZeneca, GSK, Janssen and Roche, Y.K. Onno Teng has received grants from CSL Vifor and GSK, Jinoos Yazdany has received consulting fees from AstraZeneca, Pfizer and UCB, and has received grants from Aurinia, BMS Foundation and Gilead.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (