

Background: Persistent inflammation in patients with psoriatic arthritis (PsA) can lead to permanent structural damage and negative impacts on physical function and quality of life [1]. Prevention of structural damage is a key treatment goal in PsA [2]. Bimekizumab (BKZ) is a humanised IgG1 monoclonal antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A; minimal changes in radiographic progression have previously been observed in patients with active PsA treated with BKZ up to 1 year [3].
Objectives: To assess radiographic progression at 2 years with BKZ treatment in biologic disease-modifying antirheumatic drug (bDMARD)-naïve patients with active PsA in a phase 3 study and its open-label extension (OLE).
Methods: BE OPTIMAL (NCT03895203) assessed subcutaneous BKZ 160 mg every 4 weeks (Q4W) in patients with PsA; the study was placebo (PBO)-controlled to Week 16, at which time PBO patients switched to BKZ (PBO/BKZ). BE OPTIMAL included a reference arm (subcutaneous adalimumab [ADA] 40 mg every 2 weeks); these patients switched to BKZ at Week 52 (ADA/BKZ) with no washout between treatments. At Week 52, patients could enrol in BE VITAL (NCT04009499), an ongoing OLE, in which all patients received BKZ 160 mg Q4W. Radiographic progression was assessed on plain radiographs of the hands and feet using the van der Heijde modified Total Sharp Score (vdHmTSS; score range: 0–528, with higher scores representing greater damage), quantifying the extent of joint damage based on erosions and joint space narrowing (JSN). Radiographs were read centrally and independently by two experienced readers, blind to treatment assignment and time course of the films; readings were adjudicated by a third reader in the event of disagreement. Readings for the 2-year campaign were taken at baseline and Week 104. At Week 104, PBO/BKZ, BKZ-randomised and ADA/BKZ patients had received 88, 104 and 52 weeks of BKZ treatment, respectively. Data reported for patients in the overall radiographic set and a subgroup of patients at higher risk of progression (at-risk set; high-sensitivity C-reactive protein levels [hs-CRP] ≥6 mg/L and/or ≥1 bone erosion at baseline): mean change from baseline (CfB) in vdHmTSS, cumulative probability of vdHmTSS CfB and the proportion of patients with no radiographic progression (vdHmTSS CfB ≤0.5 and ≤0.0) at 2 years of total therapy. Additional outcomes include mean CfB in the vdH Erosions and JSN sub-scores. Data are reported as observed case for all patients with radiographs available at Week 104.
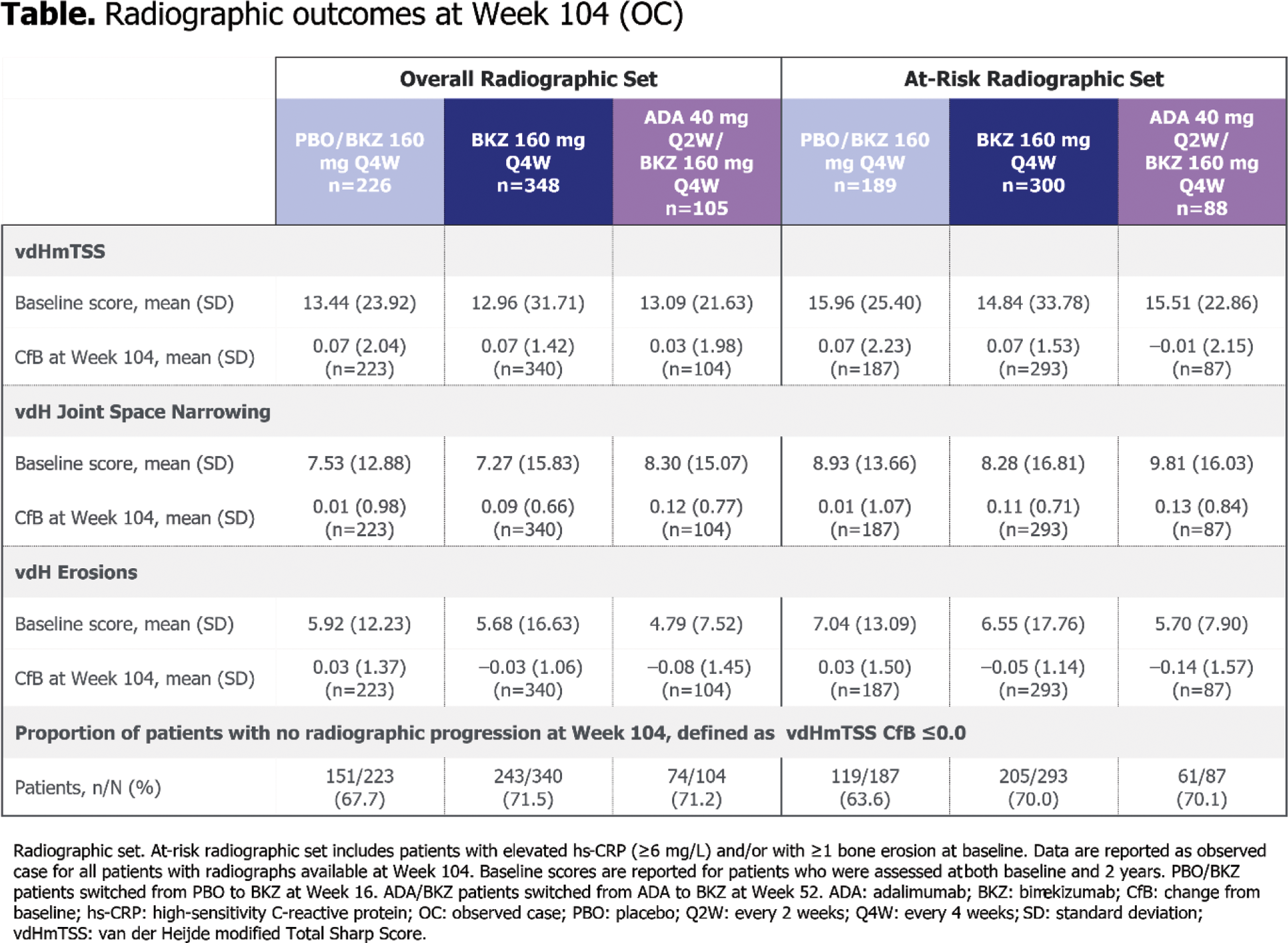
Results: Of the 852 patients randomised at baseline, 679 patients in the overall radiographic set (226 PBO/BKZ, 348 BKZ, 105 ADA/BKZ) and 577 patients in the at-risk set (189 PBO/BKZ, 300 BKZ, 88 ADA/BKZ) had radiographs taken at baseline and during the OLE. Patients had similar vdHmTSS scores at baseline across treatment arms (Table 1). Radiographic progression was minimal to Week 104 in both the overall radiographic set (vdHmTSS mean CfB [standard deviation]: 0.07 [2.04] PBO/BKZ, 0.07 [1.42] BKZ, 0.03 [1.98] ADA/BKZ) and the at-risk set (0.07 [2.23] PBO/BKZ, 0.07 [1.53] BKZ, –0.01 [2.15] ADA/BKZ; Table 1). High proportions of patients experienced no radiographic progression (vdHmTSS CfB ≤0.5) at Week 104, both in the overall radiographic set (79.4% PBO/BKZ, 84.4% BKZ, 83.7% ADA/BKZ) and the at-risk set (75.9% PBO/BKZ, 82.6% BKZ, 82.8% ADA/BKZ; Figure 1A). The majority of patients had minimal change in vdHmTSS score at 2 years (Figure 1B). There were minimal changes in mean vdH Erosions and JSN scores at 2 years across all treatment arms, in both the overall radiographic set and the at-risk set (Table 1).
Conclusion: Inhibition of radiographic progression was observed at 2 years with BKZ treatment in bDMARD-naïve patients with active PsA, with most patients experiencing no radiographic progression, regardless of original randomisation group.
REFERENCES: [1] van der Heijde D. Arthritis Res Ther 2020;3:18;
[2] Gossec L. Ann Rheum Dis 2024;83:706–19;
[3] Ritchlin CT. Ann Rheum Dis 2023;82:1404–14.
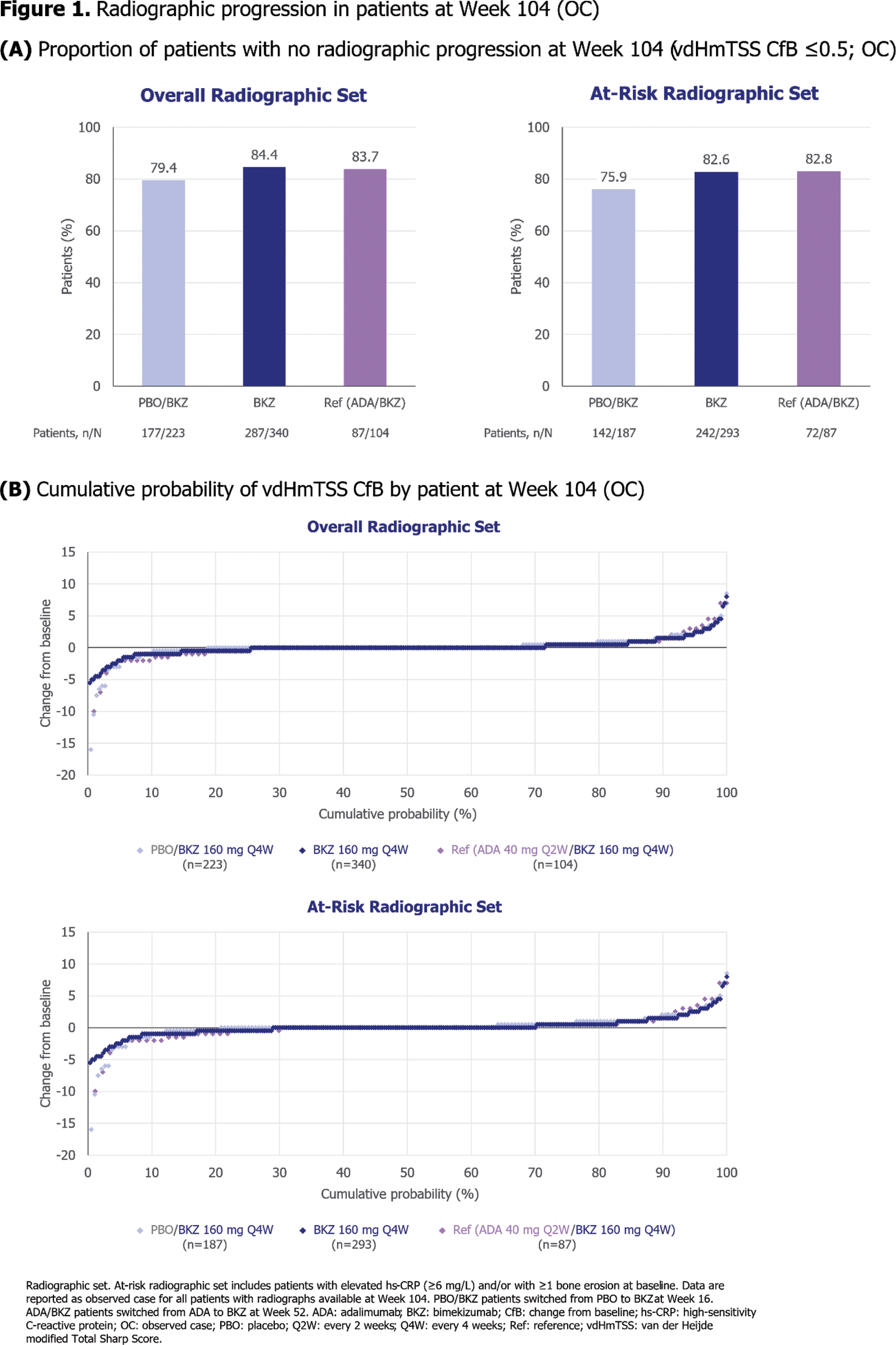
Table 1.
Acknowledgements: Funded by UCB. Medical writing support provided by Costello Medical and funded by UCB.
Disclosure of Interests: Laura C. Coates Speaking fees from AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, medac, Novartis, Pfizer and UCB, Consultant for AbbVie, Amgen, BMS, Boehringer Ingelheim, Celgene, Domain, Eli Lilly, Galapagos, Gilead, Janssen, Moonlake Immunotherapeutics, Novartis, Pfizer and UCB, Grant/research support from from AbbVie, Amgen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB, M Elaine Husni Advisory board member and consultant for AbbVie, Amgen, BMS, Eli Lilly, Janssen, Novartis, Pfizer and UCB, Mitsumasa Kishimoto Speakers bureau fees from AbbVie, Amgen, Asahi-Kasei Pharma, Ayumi Pharma, BMS, Chugai, Daiichi Sankyo, Eisai, Gilead, Janssen, Lilly, Novartis, Tanabe-Mitsubishi and UCB, Consultant for AbbVie, Amgen, Asahi-Kasei Pharma, Ayumi Pharma, BMS, Chugai, Gilead, Janssen, Lilly, Novartis, Takeda, Tanabe-Mitsubishi and UCB, Proton Rahman Consulting fees from Abbott, AbbVie, Amgen, BMS, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB, Research grants from Janssen and Novartis, Philipp Sewerin Speakers bureau fees from AXIOM Health, Amgen, AbbVie, Biogen, BMS, Celgene, Chugai Pharma Marketing Ltd./Chugai Europe, Deutscher Psoriasis-Bund, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly/Lilly Europe/Lilly Global, medi-login, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Spirit Medical Communication, Swedish Orphan Biovitrum and UCB, Consultant for AXIOM Health, Amgen, AbbVie, Biogen, BMS, Celgene, Chugai Pharma Marketing Ltd./Chugai Europe, Deutscher Psoriasis-Bund, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly/Lilly Europe/Lilly Global, medi-login, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Spirit Medical Communication, Swedish Orphan Biovitrum and UCB, Grant/research support from AXIOM Health, Amgen, AbbVie, Biogen, BMS, Celgene, Chugai Pharma Marketing Ltd./Chugai Europe, Deutscher Psoriasis-Bund, Gilead Sciences, Hexal Pharma, Janssen-Cilag, Johnson & Johnson, Lilly/Lilly Europe/Lilly Global, medi-login, Mediri GmbH, Novartis Pharma, Onkowissen GmbH, Pfizer, Roche Pharma, Rheumazentrum Rhein-Ruhr, Sanofi-Genzyme, Spirit Medical Communication, Swedish Orphan Biovitrum and UCB, Enrique R. Soriano Honoraria for advice and lectures on behalf of AbbVie, BMS, Eli Lilly, GSK, Johnson & Johnson, Novartis, Pfizer, Raffo and UCB, Grants for research and clinical trials on behalf of AbbVie, BMS, Eli Lilly, GSK, Johnson & Johnson, Novartis, Pfizer, Raffo and UCB, Barbara Ink Shareholder of AbbVie, GSK and UCB, Employee of UCB, Rajan Bajracharya Shareholder of UCB, Employee of UCB, Jason Coarse Shareholder of UCB, Employee of UCB, Philip J. Mease Speakers bureau fees from AbbVie, Amgen, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB, Consulting fees from AbbVie, Acelyrin, Amgen, BMS, Cullinan, Eli Lilly and Company, GSK, Inmagene, Janssen, Moonlake Pharma, Novartis, Pfizer, Takeda, UCB and Ventyx, Research grants from AbbVie, Acelyrin, Amgen, BMS, Eli Lilly and Company, Janssen, Novartis, Pfizer, Sana and UCB, Peter Nash Speakers bureau fees from AbbVie, AstraZeneca, BMS, Janssen, Lilly, Novartis, Pfizer and UCB, Consultant for AbbVie, AstraZeneca, BMS, Janssen, Lilly, Novartis, Pfizer, Servatus, UCB and Xencor, Grant/research support from AbbVie, Amgen, AstraZeneca, BMS, Janssen, Lilly, Novartis, Pfizer, Servatus, UCB and Xenco.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (