

Background: Adult Still’s disease is a systemic inflammatory disorder characterized by spiking fever, sore throat, skin rash, arthralgia, and neutrophilic leukocytosis [1]. Pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-18, and interferon-γ (IFN-γ), are involved through a complex cytokine loop. Recently, biological drugs targeting specific cytokine signals, such as IL-6 and IL-1, have shown efficacy in controlling disease activity of Still’s disease [1]. Tocilizumab, an anti-IL-6 receptor inhibitor, has shown efficacy in controlling disease activity of Still’s disease [2], while the profiles of inflammatory cytokine levels during the treatment and the cytokine patterns for response to tocilizumab remain poorly understood.
Objectives: To predict efficacy of an IL-6 receptor inhibitor in patients with adult Still’s disease through analysing inflammatory cytokine profiles.
Methods: This is a subanalysis of the 52-week, randomised, double-blind, placebo-controlled trial of tocilizumab in patients with adult Still’s disease (UMIN000012987) [2]. In the trial, patients diagnosed with adult Still’s disease who had shown inadequate response to more than two weeks of glucocorticoids (at least 0.5 mg/kg of prednisolone) were enrolled. Patients were randomised to receive either intravenous tocilizumab at a dose of 8 mg/kg or placebo every two weeks, and after this initial 12-week period, all patients received intravenous tocilizumab until week 52. IL-1β, IL-6, IL-6 receptor, IL-8, IL-12, IL-18, granulocyte macrophage colony stimulating factor (GM-CSF), TNFα, and IFN-γ, were measured at weeks 0, 4, 6, 8, 10, 12, 28, and 52, and its pattern and profiles were analysed based on the response to tocilizumab. We employed the achievement of ACR50 response and no clinical points on the SFS at week 52 under biweekly intravenous tocilizumab as a response.
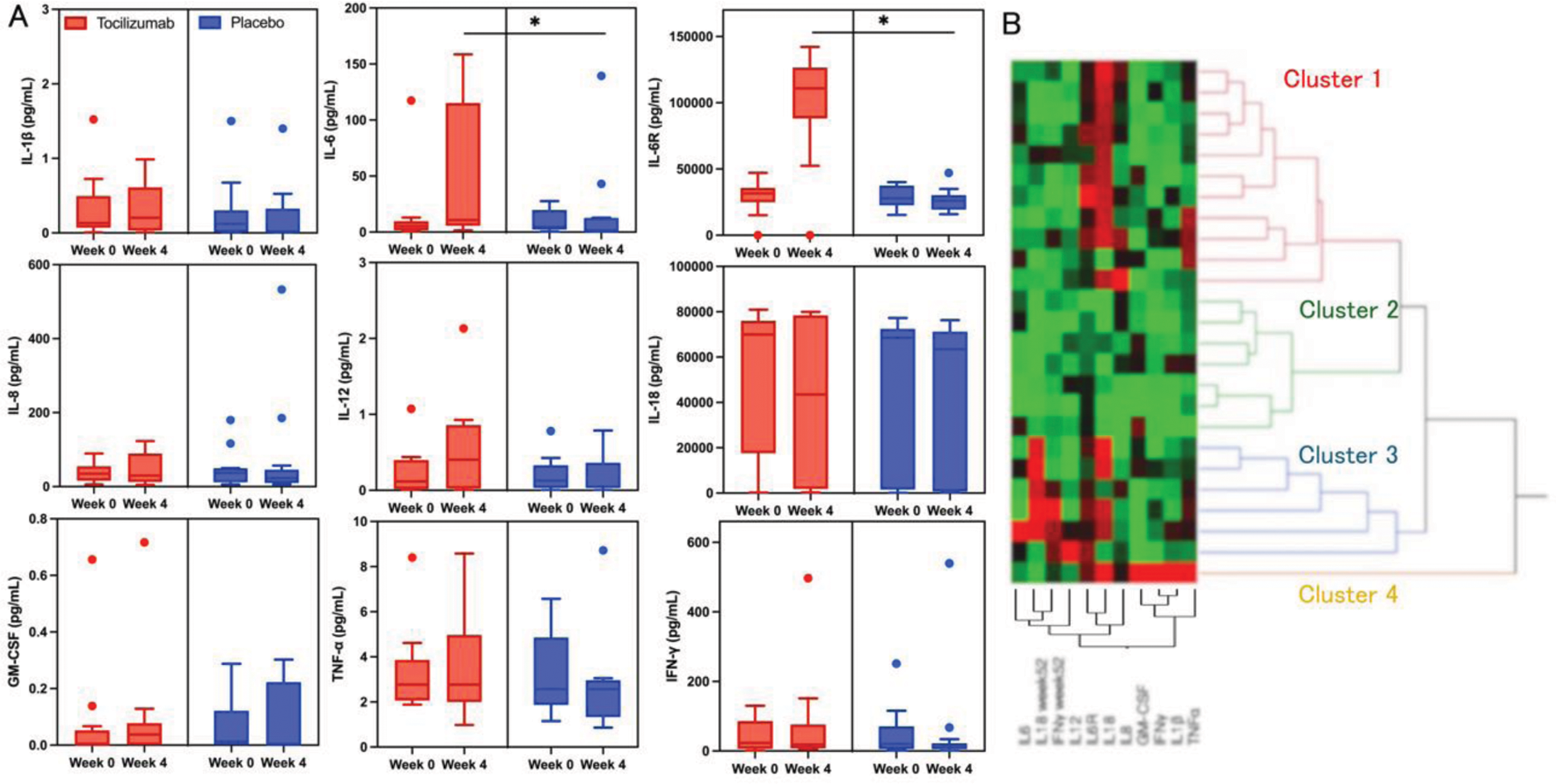
Results: A total of 26 patients (13 in the tocilizumab group and 13 in the placebo group) were enrolled. Before tocilizumab treatment, IL-6 levels were correlated with disease activity score with 28 joints (r=0.67, p<0.01), and IL-1β, IL-6, and IL-18 levels tended to be correlated with systemic feature score (r=0.34, p=0.09; r=0.4, p=0.048; r=0.4, p=0.05, respectively). IL-6 and IL-6 receptor levels were significantly elevated after tocilizumab initiation, while the other cytokines showed no significant difference compared to placebo (Figure 1A). Baseline levels of IFN-γ and IL-1β were significantly higher in non-responders compared to responders (28.49 vs 5.65 pg/mL, p=0.03; 0.26 vs 0.04 pg/mL, p=0.048), and IFN-γ and IL-18 levels at week 52 remained high in non-responders (15.56 vs 7.03 pg/mL, p=0.02; 5924 vs 392 pg/mL, p=0.02). When we divided patients into four groups according to the high/low IL-1β and IFN-γ levels with a cut-off of IL-1β > 0.07 pg/mL and IFN-γ > 7.80 pg/mL, 85% of both high patients were non-responders, whereas all patients with both low were responders. Cluster analysis classified the patients into four subgroups (Figure 1B). Cluster 2 exhibited significantly low levels of baseline IL-18 and IL-6 receptor compared to the other clusters. Clusters 1 and 3 showed largely similar baseline cytokines but were different in terms of IL-18 and IFN-γ levels at week 52. Cluster 4 showed remarkably high levels of multiple cytokines. Response rates to tocilizumab were 54.5%, 57.1%, 0%, and 0% in Clusters 1, 2, 3, and 4, respectively.
Conclusion: The effect of tocilizumab is limited to the IL-6 signaling pathway in adult Still’s disease. Patients who did not respond to tocilizumab exhibited distinct cytokine profile which pivots IFN-γ axis. These findings highlight the role of IL-6 inhibition in adult Still’s disease and shed light on its personalised treatment strategies.
REFERENCES: [1] Fautrel B, et al. Ann Rheum Dis. 2024; ard-2024-225851.
[2] Kaneko Y, et al. Ann Rheum Dis. 2018;77: 1720–1729.
A and 1B
Cytokine levels at baseline and week 4 demonstrated a significant elevation of IL-6 and IL-6 receptor in the tocilizumab group compared to the placebo group. * p < 0.05 by the Mann-Whitney U test (A). Hierarchical clustering of Adult Still’s disease cases divided patients into four subgroups, each represented by the colors red, green, blue, and yellow, respectively (B).
Acknowledgements: NIL.
Disclosure of Interests: Koji Suzuki: None declared, Hideto Kameda AbbVie Japan GK, Asahi KASEI Pharma Co., Ltd., Bristol Myers Squibb Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Mitsubishi-Tanabe Pharma Co., Ltd., Janssen Pharmaceutical K.K., and UCB Japan Co. Ltd, Asahi KASEI Pharma Co., Ltd., Taisho Pharmaceutical Co., Ltd., and Pfizer Japan Inc., Kei Ikeda AbbVie, Asahi Kasei, AstraZeneca, Bristol-Myers Squibb, Chugai, Eisai, Eli Lilly, Gilead Sciences, Janssen, Mitsubishi-Tanabe, Novartis, Pfizer, and UCB, Mitsubishi-Tanabe, Tomonori Ishii AbbVie Japan GK, Asahi KASEI Pharma Co., Ltd., Astellas Pharma Inc., AstraZeneca plc., Ayumi, Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company,GlaxoSmithKline K.K., Mitsubishi-Tanabe Pharma Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd.,Janssen Pharmaceutical K.K., and UCB Japan Co. Ltd., Asahi KASEI Pharma Co., Ltd, Kosaku Murakami AbbVie G.K., Asahi Kasei Pharma, Astellas Pharma Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co.,Ltd, Eisai Co., Ltd, Eli Lilly Japan K.K., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Ono Pharmaceutical Co., Pfizer Inc. and UCB Japan Co., Hyota Takamatsu: None declared, Yoshiya Tanaka Abbvie, Eisai, Chugai, Eli-Lilly, Behringer-Ingelheim, GlaxoSmithKline, Taisho, AstraZeneca, Daiichi-Sankyo, Gilead, Pfizer, UCB, Asahi-kasei, Astellas, received research grants from Behringer-Ingelheim, Taisho, Chugai., Takayuki Abe: None declared, Tsutomu Takeuchi AbbVie GK, Chugai, Eli Lilly Japan, Eisai, Gilead Sciences Inc, Pfizer Japan Inc, Taisho Pharma, AbbVie GK, Eli Lilly Japan, Gilead Sciences, Inc. Mitsubishi-Tanabe, Taisho Pharma, Yuko Kaneko AbbVie Japan GK, Asahi KASEI Pharma Co., Ltd., Astellas Pharma Inc., AstraZeneca plc., Ayumi, Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Gilead Sciences, Inc., GlaxoSmithKline K.K., Mitsubishi-Tanabe Pharma Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., and UCB Japan Co. Ltd., Asahi KASEI Pharma Co., Ltd., Boehringer Ingelheim Japan, Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi-Tanabe Pharma Co.,Ltd, and Pfizer Japan Inc.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (