

Background: Anakinra is recommended after failure of colchicine, corticosteroids and non-steroid anti-inflammatory drugs (NSAID) in the treatment of gout and calcium pyrophosphate deposition (CPPD) flares. Nevertheless, the use of these first-line treatments is often limited because of renal and cardiovascular comorbidities that are more frequently observed in patients with crystal-related diseases (CRD). The safety of anakinra was widely studied in rheumatoid arthritis but poorly in crystal arthritis.
Objectives: To assess efficacy and safety of anakinra in acute CRD, and to identify risk factors associated with treatment failure and infections.
Methods: In this retrospective cohort study, we identified patients treated by anakinra for an acute CRD at a tertiary care hospital between January 2011 and July 2024, using an electronic query. We confirmed CRD diagnosis according to the presence of crystals in the synovial fluid or specific ultrasonography features, and collected clinical and biological data by medical record review. Efficacy was defined by the resolution of CRD-flare following anakinra initiation. Potential adverse events (AEs) were collected up to one month after anakinra initiation. Associations with anakinra efficacy and risk of infection within 1-month of treatment were tested using univariable and multivariable analyses. The multivariable model included variables with an unadjusted P-value < 0.10.
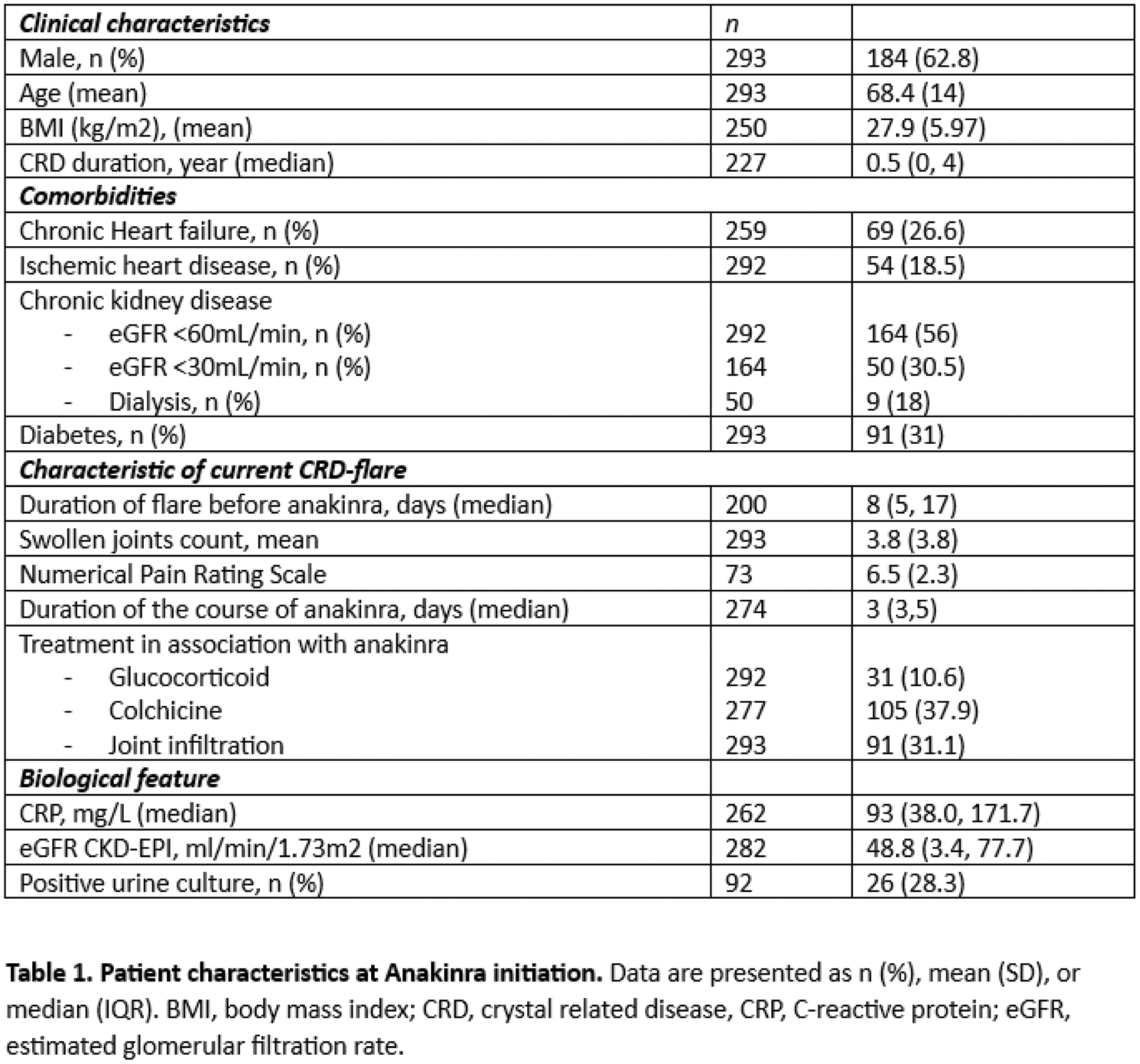
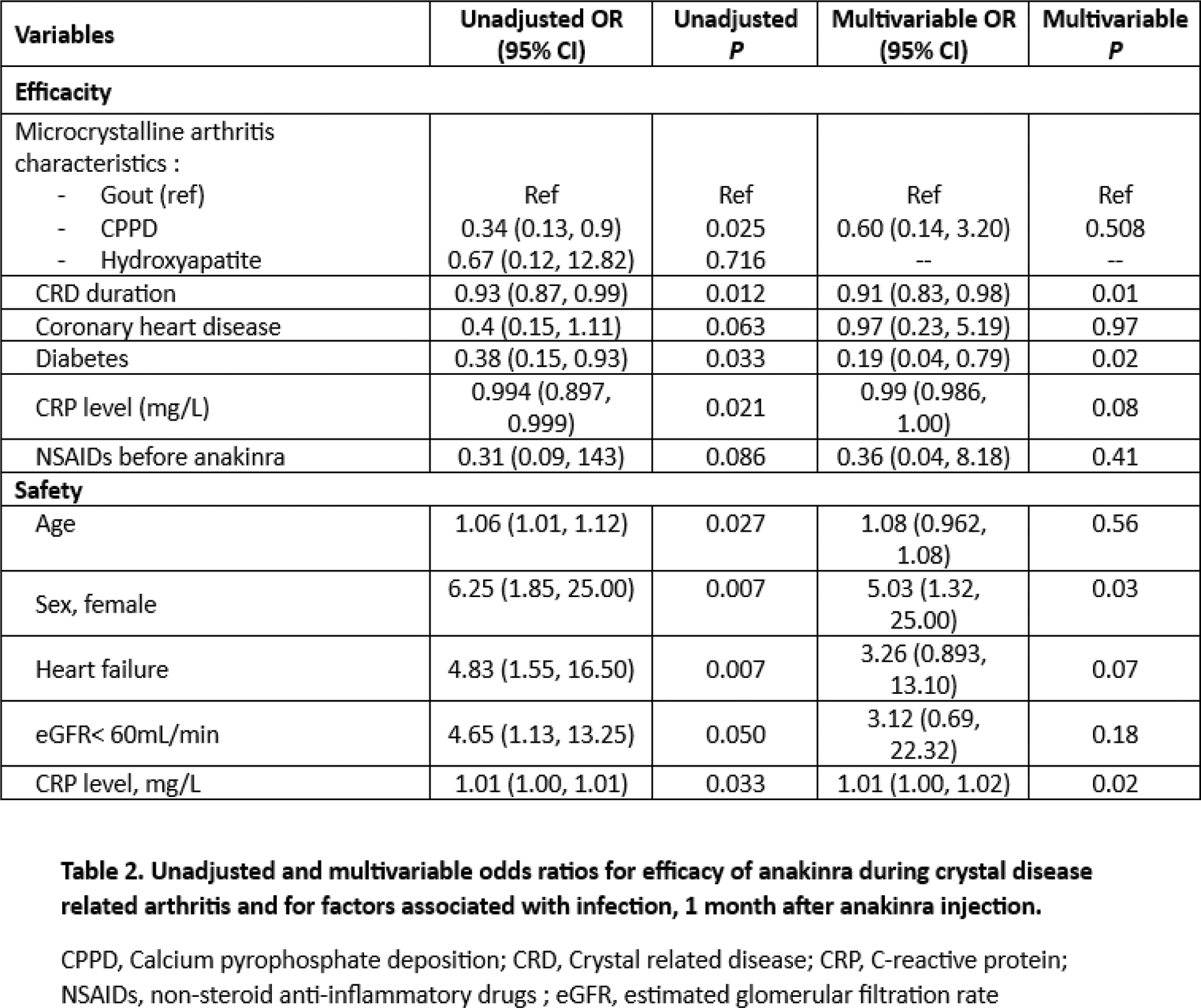
Results: We included 293 patients: 184 men (62.8%), mean age at CRD flare 68.4 ± 14 years, (Table 1), with 212 (72.4%) gout, 54 (18.3%) CPPD arthritis, 12 (4.1%) hydroxyapatite crystal disease (HCD) and 15 (5.2%) presenting both gout and CPPD or gout and HCD features. Crystals were observed in the synovial fluid for 60.8% of patients with gout and 61.7% for those with CPPD. A history of diabetes was observed in 91 patients (31%), ischemic heart disease in 54 (18.5%), chronic heart failure (CHF) in 69 (26.6%), chronic kidney disease in 164 (56.6%) with 30.5% having a severe renal failure defined by eGFR <30mL/min, solid organ transplant treated with immunosuppressive drugs in 16 (5.4%), and other cause of immunodepression 55 (18.8%). Anakinra was prescribed as a first-line treatment for CRD flare in 169 patients (56.8%). The median duration of CRD-flare before anakinra initiation was 8 days (5, 17). After a median treatment duration of 3 days (3-5), efficacy of anakinra was observed in 266/287 patients (92.7%). Among the 21 partial or not responder to treatment: 57% were men, mean age 70.4±12.5 years old. In the univariate analysis, patient who showed less efficacy of anakinra had more CPPD (38.1% vs 17.3%), a longer CRD duration (3 years [0-10] vs 0.5 [0-3]), more diabetes (52% vs 29%), a higher baseline CRP (112mg/L [71-230] vs 83 [30-153]), and received more frequently NSAIDs (14% vs 4.9%) and febuxostat (35% vs 14%). Use of colchicine and glucocorticoid did not differ. In the multivariate analysis, CRD duration and a history of diabetes were independently associated with anakinra failure: OR 0.91, 95% CI (0.83- 0.98) and OR 0.19, 95% CI (0.04-0.79), respectively. (Table 2) A 1-month follow-up was available for 226 patients. An infection was observed for 13 (5.7%), after a median time of 11 (7-16) days after anakinra initiation (4 urinary tract infection, 3 E.Coli bacteriemia, 3 pneumonitis, 2 COVID-19, 1 spondylodiscitis). 92 patients had a urine culture before anakinra initiation (71.7% sterile, 28.3% asymptomatic bacteriuria). Patient with asymptomatic bacteriuria had more often an infection after anakinra (19.2% vs 3%). In the univariate analysis, patients who developed infection were more often women (76.9% vs 35%), were older (76.7 ± 9.4 years old vs 67.7 ± 14), had more frequently an eGFR <60mL/min (61.5% vs 38.5%), more CHF history (61.5% vs 24.9%) and had higher baseline CRP level (216mg/L [152-231], vs 90.5 [38-164]). Treatment (colchicine, glucocorticoids, NSAIDs) were similar in both group. In the multivariate analysis, female sex and higher baseline CRP level were independently associated with risk of infection following anakinra, OR 5.03, 95% CI (1.32-25.00) and OR 1.01, 95% CI (1.00-1.02) for each increase of 1mg/L, respectively. (Table 3). During follow-up, a neutrophils blood count <1 G/L was observed in 8/226 patients (3.5%), that did not exceed 0.5 G/L and patients did not develop infection. No case of skin reaction was reported.
Conclusion: In our retrospective study, efficacy of anakinra was observed in more than 90% of patients experiencing a CRD- flare. Diabetes and longer duration of CRD were associated with a lack of efficacy of anakinra. Female sex and higher CRP level were associated with an increased risk of infection during the month following treatment. Whether the treatment of asymptomatic bacteriuria before initiating anakinra prevent the development of urinary tract infection remains to be determined.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (