

Background: Psoriatic arthritis (PsA) is a heterogeneous, chronic inflammatory condition, with low remission rates, divergence of tissue response to therapy, and inadequate outcomes aggravated by co-morbidities. A proportion of patients with PsA have disease which is refractory or resistant to available therapies with significant health and socioeconomic impact for individuals and health care systems.
Objectives: To develop evidence-based consensus definitions of Difficult-to-Manage (D2M) and Treatment-Refractory (TR) PsA.
Methods: A multidisciplinary international EULAR task force (TF) comprising 27 members was established and the EULAR standardized operating procedures were followed.
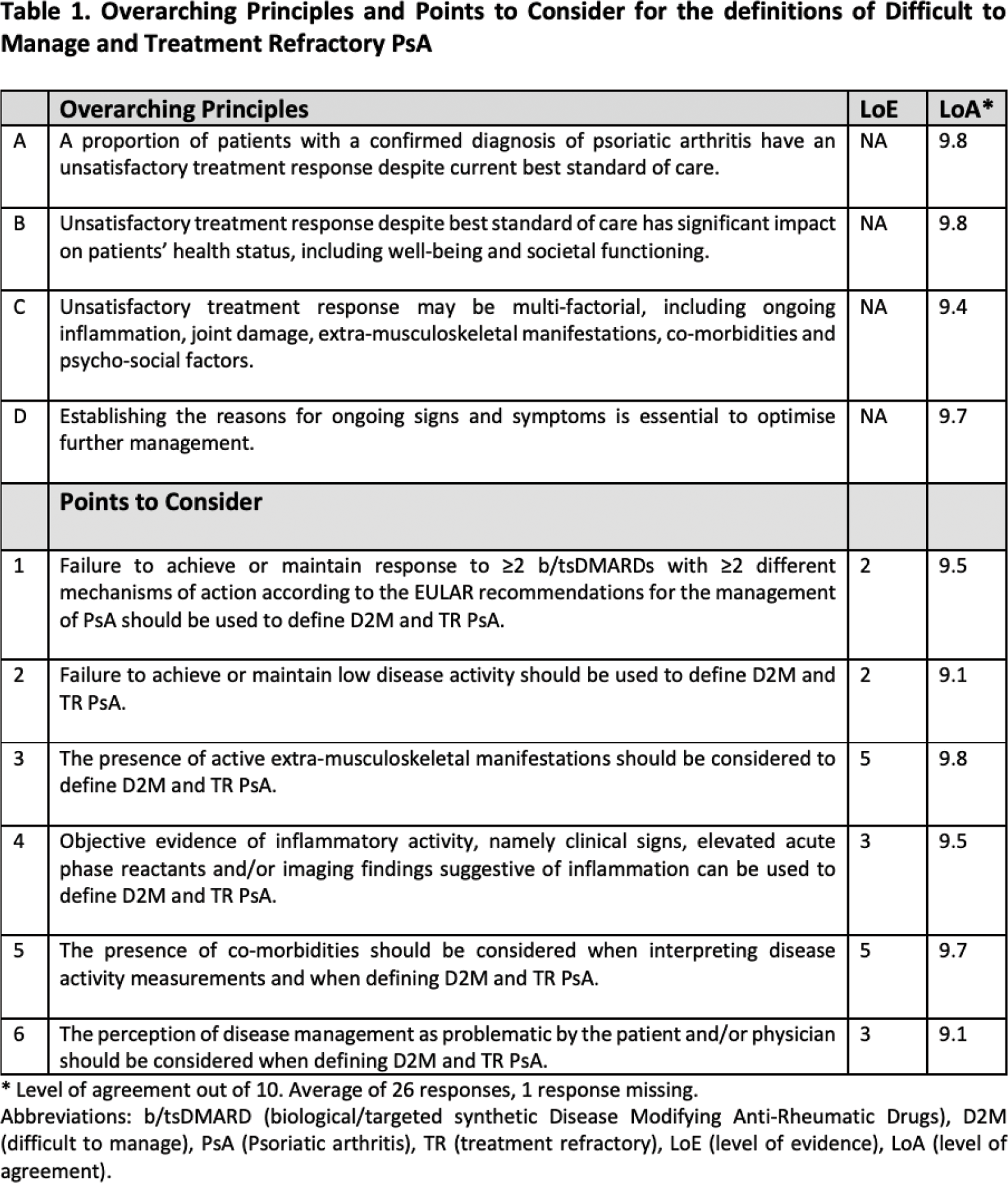
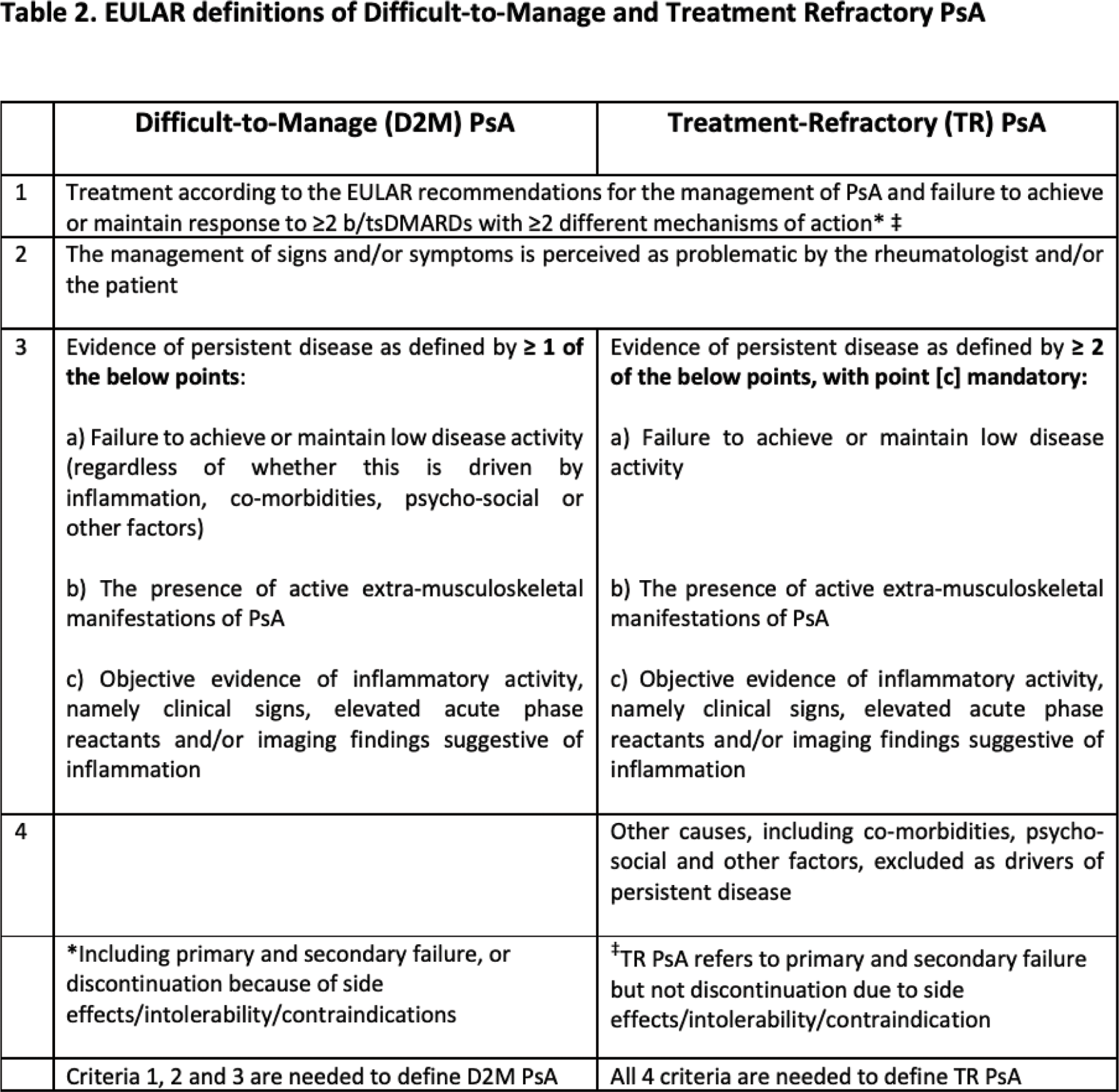
Results: A systematic literature review (92 articles, 6 abstracts) identified relevant concepts and possible criteria. Four overarching principles (OAPs) address the subset of patients with PsA with an unsatisfactory treatment response despite best standard of care and for which the causes are likely multi-factorial (Table 1). Six Points to consider (PtC) highlight relevant criterion including failure to achieve or maintain response to ≥2 b/tsDMARDs with ≥2 different mechanisms of action; management of signs and symptoms perceived as problematic by the rheumatologist and/or the patient, and evidence of persistent disease activity in the presence of extra-musculoskeletal manifestations and/or co-morbidities and/or objective evidence of inflammatory activity (Table 1). The TF voted to change the preferred terminology for the concept from difficult-to-treat and agreed unanimously to develop two definitions: 1) D2M PsA, a wider concept including drivers such as inflammation, co-morbidities, psycho-social or other factors and a subgroup of 2) TR PsA defined by persistent disease activity and objective evidence of active inflammation (Table 2).
Conclusion: EULAR proposes two consensus definitions to identify a D2M PsA population including a TR subgroup. These definitions should now be applied in well-designed observational and interventional studies to characterise the phenotypes of these two populations, test interventions and guide further research in order to understand disease pathogenesis and improve care.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Helena Marzo-Ortega AbbVie, Biogen, Eli-Lilly, Janssen, Novartis, Pfizer, Takeda and UCB, AbbVie, Eli-Lilly, Janssen, Moonlake, Novartis, Pfizer and UCB, Janssen, Novartis, Pfizer and UCB, Stephanie R Harrison Janssen and Novartis, George Fragoulis AbbVie, Amgen, Boerhinger Ingelheim, Farran, GSK, Janssen, MSD, Novartis, Pfizer, UCB, AbbVie, Amgen, Boerhinger Ingelheim, Farran, GSK, Janssen, MSD, Novartis, Pfizer, UCB, Xabier Michelena Janssen and Novartis, Cristina Macía-Villa AbbVie, Amgen, Lilly, Janssen, Novartis, Pfizer and UCB, AbbVie, Amgen, Lilly, Janssen, Novartis, Pfizer and UCB, UCB, Sibel Aydin AbbVie, Fresenius Kabi, Janssen, Lilly, Novartis, Pfizer and UCB, AbbVie, Fresenius Kabi, Janssen, Lilly, Novartis, Pfizer and UCB, AbbVie, Fresenius Kabi, Janssen, Lilly, Novartis, Pfizer and UCB, Andra Bălănescu AbbVie, Amgen, AlphaSigma, Astra-Zeneca, Angellini, Biogen, BMS, Berlin-Chemie, Boerhinger-Ingelheim, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Teva, UCBA, Zentiva, AbbVie, Amgen, AlphaSigma, Astra-Zeneca, Angellini, Biogen, BMS, Berlin-Chemie, Boerhinger-Ingelheim, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Teva, UCBA, Zentiva, Heidi Bertheussen: None declared, Christine Bundy AbbVie, Almirall, Beiersdorf, Galapagos, Janssen, Novartis, Pfizer and UCB, AbbVie, Almirall, Beiersdorf, Galapagos, Janssen, Novartis, Pfizer and UCB, AbbVie, Almirall, Beiersdorf, Galapagos, Janssen, Novartis, Pfizer and UCB, Maria Sole Chimenti AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer and UCB, AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer and UCB, AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer and UCB, Paolo Gisondi Amgen, AbbVie, Almirall, Boehringer Ingelheim Janssen, Eli-Lilly, Novartis, Pfizer and UCB, Amgen, AbbVie, Almirall, Boehringer Ingelheim Janssen, Eli-Lilly, Novartis, Pfizer and UCB, Bente Glintborg AbbVie, AlfaSigma, Eli-Lilly, Pfizer and Sandoz, Laure Gossec AbbVie, Amgen, BMS, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, Stada, UCB, AbbVie, Biogen, Lilly, Novartis, UCB, Umut Kalyoncu: None declared, Ennio Lubrano Abbvie, Janssen, Lilly, Pfizer, UCB, Abbvie, Janssen, Lilly, Pfizer, UCB, György Nagy Astra Zeneca, AbbVie, Boehringer, GSK, Lilly, Novartis, Pfizer, MSD, Roche, Miltenyi, Richter, SOBI, Swixx and UCB, Astra Zeneca, AbbVie, Boehringer, GSK, Lilly, Novartis, Pfizer, MSD, Roche, Miltenyi, Richter, SOBI, Swixx and UCB, Wendy Wagenaar: None declared, Luis Puig Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius-Kabi, Horizon, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, Samsung-Bioepis, STADA, Sun-Pharma, Takeda, and UCB, Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius-Kabi, Horizon, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, Samsung-Bioepis, STADA, Sun-Pharma, Takeda, and UCB, Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius-Kabi, Horizon, J&J Innovative Medicine, Leo-Pharma, Lilly, Novartis, Pfizer, Samsung-Bioepis, STADA, Sun-Pharma, Takeda, and UCB, Rubén Queiro AbbVie, Amgen, Janssen, Novartis, Pfizer, UCB and Lilly, AbbVie, Amgen, Janssen, Novartis, Pfizer, UCB and Lilly, Abbvie, Novartis, Janssen, UCB, Proton Rahman Abbott, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB, Janssen and Novartis, Russka Shumnalieva AstraZeneca, Novartis, Abbvie, Janssen, AstraZeneca, Novartis, Abbvie, Janssen, Enrique R. Soriano AbbVie, Amgen, BMS, Elea, Janssen, Lilly, Novartis, _Pfizer, UCB, AbbVie, Amgen, BMS, Elea, Janssen, Lilly, Novartis, _Pfizer, UCB, AbbVie, Amgen, BMS, Elea, Janssen, Lilly, Novartis, _Pfizer, UCB, Filip van den Bosch Abbvie, Alfasigma, Amgen, Eli Lilly, Fresenius Kabi, Janssen, Novartis and UCB, Abbvie, Alfasigma, Amgen, Eli Lilly, Fresenius Kabi, Janssen, Novartis and UCB, Marleen G.H. van de Sande Abbvie, Janssen, Eli Lilly, Novartis, UCB, Abbvie, Janssen, Eli Lilly, Novartis, UCB, Janssen, Novartis and UCB, Alexandre Sepriano AbbVie, Novartis, UCB and Lilly, AbbVie, Novartis, UCB and Lilly, Pedro Machado Abbvie, Abcuro, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, Abbvie, Abcuro, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, Stefan Siebert AbbVie, Amgen, AstraZeneca, Janssen, Novartis, Pfizer, Syncona, Teijin Pharma and UCB, AbbVie, Amgen, AstraZeneca, Janssen, Novartis, Pfizer, Syncona, Teijin Pharma and UCB, Amgen (previously Celgene), Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, GSK, Janssen and UCB.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (