

Background: Patients with systemic lupus erythematosus (SLE) undergoing immunosuppressive therapy are at increased risk of herpes zoster (HZ). Updated international guidelines now recommend that immunocompromised persons aged 18 years and older be vaccinated with the recombinant VZV vaccine.
Objectives: We aimed to assess the risk factors for HZ in patients with systemic lupus erythematosus.
Methods: This single-centre, retrospective study included all consecutive patients with SLE registered in our referral centre registry. Medical records were collected at the time of clinical visit between July and December 2024. Demographic, medical history, laboratory and treatment data were extracted from the electronic medical records using a standardised data collection form. Factors associated with HZ were identified.
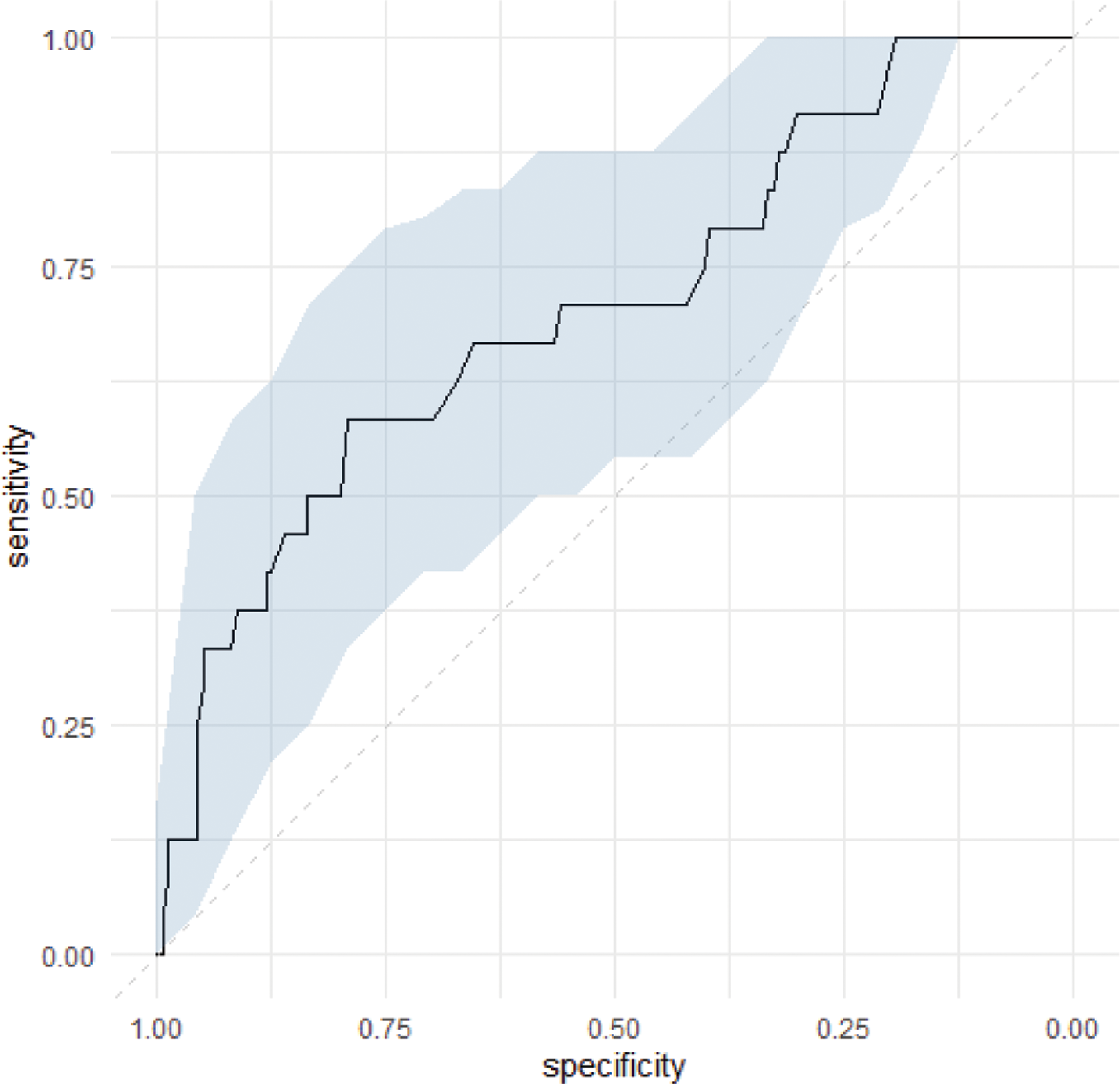
Results: A total of 224 SLE patients (female 93.8%, median age 48 [1st quartile-3rd quartile: 38;56] years) were analysed (Table 1). Of these, 30 (n=30/224, 13.4%) had HZ (Figure 1). Univariable analysis of factors associated with HZ showed that gamma globulin level (OR 0.81, 95%CI 0.70-0.92), duration of steroids treatment (OR 1.07, 95% CI 1.03-1.11), immunosuppressive (IS) drugs (OR 2.41, 95% CI 1.06-6.05), duration of IS treatment (OR 1.05, 95% CI 1.00-1.09) and biologics (OR 3.50, 95% CI 1.53-7.93) were significantly associated with HZ. In a multivariable logistic regression analysis including these 5 covariates, only gamma globulin level (OR 0.86, 95% CI 0.74-0.98) was independently associated with HZ (Table 2). The ROC curve for gamma globulin level showed a significant association with HZ (AUC = 0.71 (0.59-0.82)), with a threshold of 10.3 g/L providing the best discrimination between those with and without HZ (Figure 2).
Conclusion: A low gamma globulin level - with a cut-off of 10.3 g/L - is associated with HZ in SLE. Such a gamma globulin threshold may identify SLE patients who should be prioritised for VZV vaccination.
Characteristics of SLE patients
| Missing | all, n=224 | |
|---|---|---|
| Female Sex | 0 | 210 (93.8) |
| Age, years | 0 | 48 [38;56] |
| Duration of SLE disease, years | 9 | 15 [7;24] |
| SLEDAI score, N | 20 | 0 [0;2] |
| Arthritis | 4 | 191 (85.3) |
| Mucocutaneous | 4 | 173 (77.2) |
| Class III/IV nephritis | 3 | 73 (32.6) |
| Auto-immune cytopenia | 2 | 66 (29.5) |
| Serosal | 4 | 66 (29.5) |
| Neuropsychiatric | 4 | 26 (11.6) |
| ANA | 18 | 203 (90.6) |
| anti-dsDNA | 19 | 149 (66.5) |
| anti-SSA | 23 | 94 (42.0) |
| anti-SSB | 22 | 33 (14.7) |
| anti-RNP | 24 | 72 (32.1) |
| anti-Sm | 25 | 61 (27.2) |
| anti-PL | 21 | 64 (28.6) |
| Low C3 | 15 | 45 (20.1) |
| Low C4 | 15 | 33 (14.7) |
| Gammaglobulins, g/L | 41 | 13 [10:17] |
| Lymphocytes count, G/L | 12 | 1 [1;2] |
| GFR>60 mL/mn/1.73 m 2 | 9 | 215 (96.0) |
| Steroids | 4 | 178 (79.5) |
| Steroids daily dose, mg | 0 | 5 [5;7] |
| Length steroids, years | 15 | 6 [1;15] |
| Hydroxycholoquine | 3 | 214 (95.5) |
| Immunosupressive drugs | 3 | 121 (54.0) |
| Length IS drugs, years | 12 | 11 [5:21] |
| Biologics | 4 | 49 [21.9] |
Results are shown as median [q1;q3] or n (%).
SLEDAI, SLE Disease Activity Index; ANA, anti-nuclear antibody; GFR, glomerular filtration rate. aPL, anti-phospholipid antibodies; Immunosuppressive (IS) drugs included azathioprine (n=47), methotrexate (n=28), mycophenolate mofetil (n=78) and cyclophosphamide (n=55). Biologics included rituximab (n=24) and belimumab (n=30) and anifrolumab (n=1)
Multivariate analyses for independent predictors of herpes zoster in SLE
| OR* | IC 95% | P | OR** | IC 95% | p | |
|---|---|---|---|---|---|---|
| Female Sex | 0.92 | [0.24-6.13] | 0.919 | |||
| Age | 1.02 | [0.99-1.05] | 0.262 | |||
| Duration of SLE disease | 1.02 | [0.98-1.06] | 0.277 | |||
| SLEDAI score | 0.91 | [0.71-1.10] | 0.373 | |||
| Arthritis | 2.32 | [0.64-14.88] | 0.269 | |||
| Mucocutaneous | 1.90 | [0.69-6.70] | 0.255 | |||
| Class III/IV nephritis | 1.67 | [0.75-3.65] | 0.200 | |||
| Auto-immune cytopenia | 1.70 | [0.75-3.75] | 0.189 | |||
| Serosal | 1.98 | [0.88-4.34] | 0.090 | |||
| Neuropsychiatric | 1.17 | [0.32-3.38] | 0.782 | |||
| anti-dsDNA | 2.75 | [1.01-9.66] | 0.072 | |||
| anti-SSA | 1.00 | [0.45-2.17] | 0.991 | |||
| anti-SSB | 1.03 | [0.33-2.73] | 0.958 | |||
| anti-RNP | 0.60 | [0.24-1.38] | 0.251 | |||
| anti-Sm | 0.96 | [0.40-2.19] | 0.933 | |||
| aPL | 1.31 | [0.57-2.91] | 0.512 | |||
| Low C3 | 1.25 | [0.47-3.05] | 0.632 | |||
| Low C4 | 0.87 | [0.24-2.47] | 0.815 | |||
| Gammaglobulins | 0.81 | [0.70-0.92] | 0.002 | 0.86 | [0.74-0.98] | 0.034 |
| Lymphocytes count | 0.64 | [0.33-1.17] | 0.172 | |||
| Steroids | 2.22 | [0.73-9.66] | 0.209 | |||
| Steroids daily dose | 0.95 | [0.77-1.04] | 0.557 | |||
| Length steroids | 1.07 | [1.03-1.11] | 0.001 | 1.05 | [0.97-1.14] | 0.207 |
| Hydroxycholoquine | 0.90 | [0.15-17.39] | 0.926 | |||
| Immunosupressive drugs | 2.41 | [1.06-6.05] | 0.045 | 1.28 | [0.39-4.53] | 0.687 |
| Length IS drugs | 1.05 | [1.00-1.09] | 0.035 | 1.00 | [0.92-1.07] | 0.910 |
| Biologics | 3.50 | [1.53-7.93] | 0.003 | 1.87 | [0.62-5.37] | 0.253 |
*univariate, ** multivariate
OR, odds ratios; CI, confidence intervals; SLEDAI, SLE Disease Activity Index; aPL, anti-phospholipid antibodies; IS, immunosuppressive, PL, phospholipid
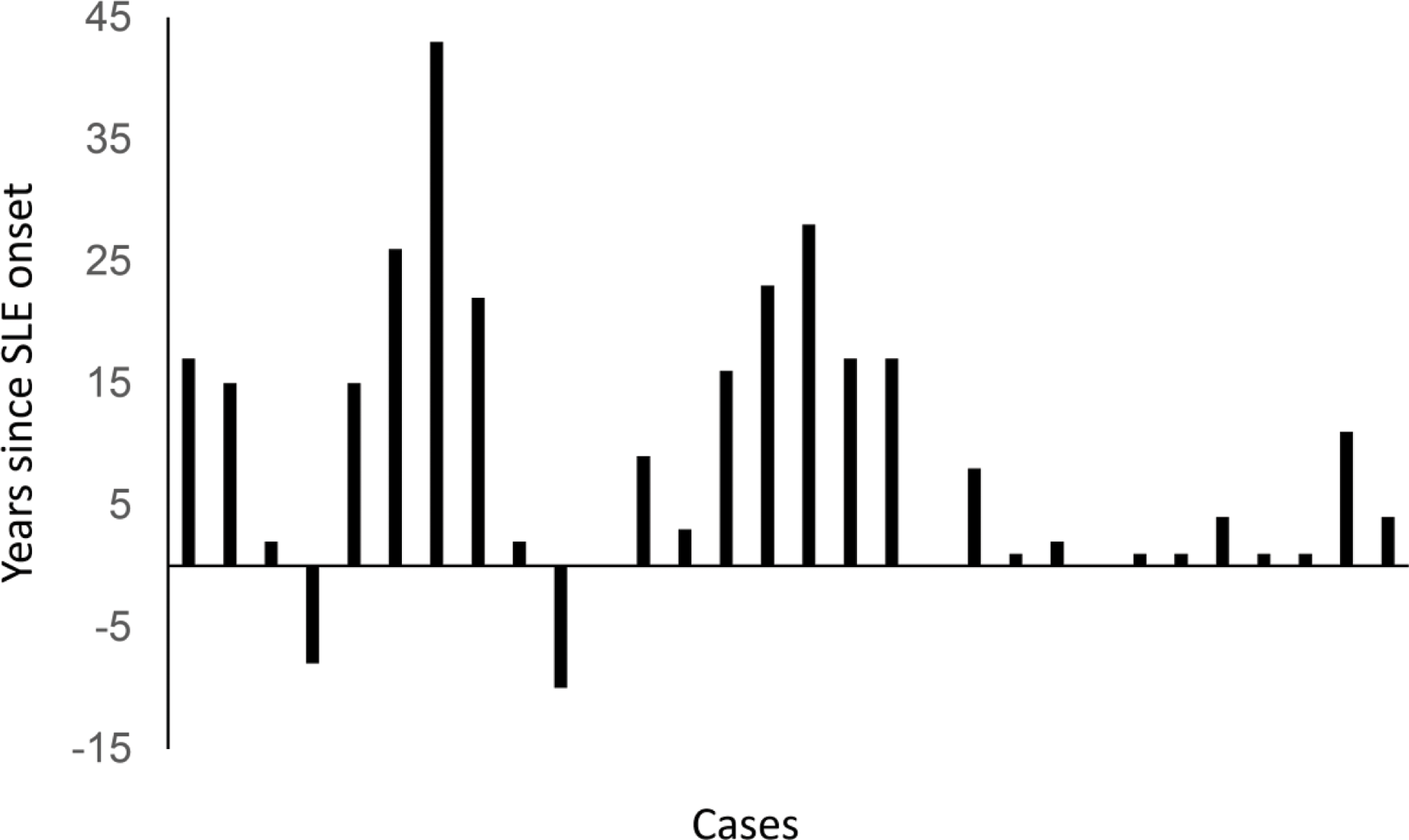
Time distribution of herpes zoster occurrence in relation to SLE onset
The median SLE duration at HZ event was 4 [1;17] years. Among the 30 patients with HZ, 2 had HZ occurrence prior the diagnosis of SLE.
Receiver operating characteristics (ROC) curve of gamma-globulin for prediction of herpes zoster in SLE
Area under the curve 0.71 (0.59-0.82) with a gamma-globulin level of 10.3 g/L showing a sensitivity of 58% and a specificity of 79% for herpes zoster
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (