

Background: Belimumab is an anti-B-cell agent approved for the treatment of lupus nephritis in 2020, based on the results of a randomized clinical trial that demonstrated its efficacy and safety when added to standard of care (SOC). Given that early treatment with immunosuppressive agents reduces disease activity, prevents flares and progression of kidney damage, and lowers cumulative glucocorticoid exposure, it is reasonable to suggest that early combination therapy with belimumab could enhance the achievement of these goals.
Objectives: In this multicentric study we aim at assessing renal response trajectories in a cohort of lupus nephritis (LN) patients receiving early combination treatment with belimumab as initial treatment atop SOC, as compared to a well characterized historical cohort of LN patients treated with SOC alone.
Methods: Adult patients followed-up across several italian lupus-referral centers, with a biopsy-proven LN classified according to the ISN/RPS 2003 criteria and receiving belimumab atop standard of care as initial treatment were included (belimumab cohort) and compared with a well-characterized LN cohort followed-up from 1990 to 2016, whose kidney biopsies were reevaluated according to the 2003 ISN/RPS criteria (historical cohort). Information of demographics, clinical features and treatment were recorded at baseline and throughout the follow-up. Propensity score matching was applied considering treatment, baseline 24h proteinuria, histological class at biopsy and activity index (AI). Rates of renal response were assessed at 6 and 12 months and were defined according to the EULAR/EDTA criteria [1] as following: complete renal response (CRR) as 24h-proteinuria < 500mg/24h with normalization/stabilization of eGFR, partial renal response (PRR) as at least 50% reduction in 24h-proteinuria since baseline with normalization/stabilization of eGFR or reduction of 24h-proteinuria <3 g if baseline 24h proteinuria was in the nephrotic range. Data were analyzed and compared using t-test for continuous values or X^2 test and Mann-Whitney U test for non-parametric variables. A logistic regression model was used to investigate predictors of response at fixed timepoints.
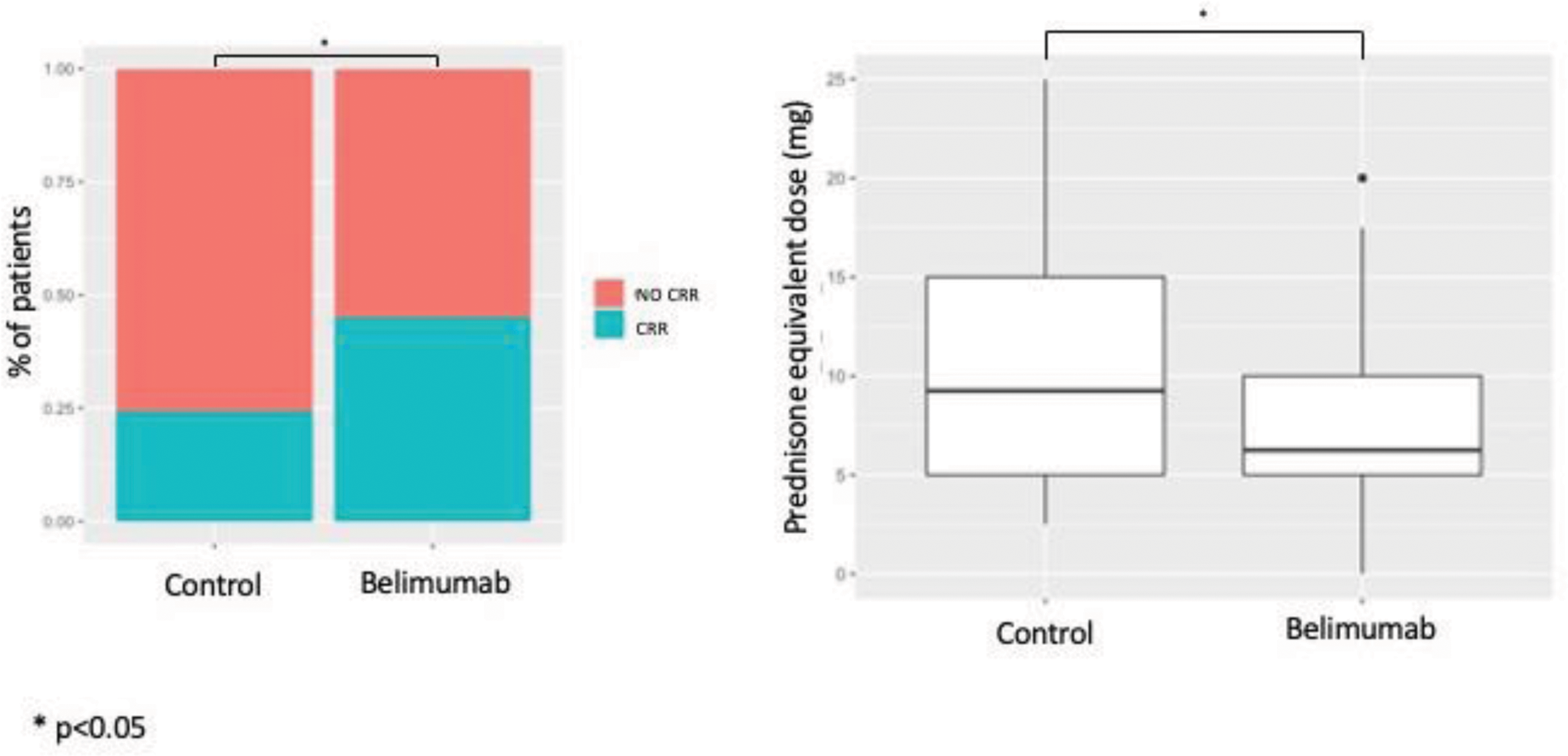
Results: Eighty-two LN patients were identified for the belimumab cohort. From the historical cohort of 303 LN patients, a cohort of 80 patients (control cohort) was selected after propensity score matching, which did not differ from the belimumab cohort in terms of baseline features (Table 1). A significantly higher proportion of patients in the belimumab cohort achieved complete renal response (CRR) at 6 months (45% vs. 24.3%, OR 2.54 95%CI 1.17-5.62; p=0.0158), whereas rates of both partial or complete response became comparable at 1 year (p=0.45) (Figure 1). Notably, patients achieving CRR in the control cohort were taking significantly higher doses of prednisolone at the time of CRR achievement (10.9 mg vs 7.55 mg, p=0.02) (Figure 1). The multivariable model was adjusted for baseline confounders. Variables tested in the model included proteinuria, age at LN onset, and initial treatment. Baseline 24h-proteinuria and early belimumab were the only significant predictors of early complete response (24h-proteinuria: OR 0.63, 95%CI 0.47-0.80, p=0.0007; belimumab: OR 2.26, 95%CI 1.06-5.33, p=0.03).
Conclusion: Combination therapy with belimumab as initial treatment portends early renal response and is associated with decreased corticosteroid intake at the time of CRR. Achieving CRR earlier in the disease course with less use of corticosteroids is an essential target in the treatment of LN patients to prevent progression of kidney damage and glucocorticoid-related damage. Our study reinforces the importance of including belimumab in the early management of LN.
Patients’ Characteristics after Propensity Score Matching.
| CONTROL COHORT n=80 | BELIMUMAB n=80 | P | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Female (%) | 68 (85) | 67 (81.7) | |
| Mean age at LN onset (sd) | 33.5 (11.71) | 36.5 (13.4) | 0.13 |
| Baseline 24h-Proteinuria (sd) | 3.42 (2.41) | 2.88 (2.63) | 0.17 |
| Baseline Creatinine (sd) | 1.19 (0.99) | 1.20 (0.91) | 0.95 |
| Baseline eGFR (sd) | 80.0 (38.6) | 83.5 (33.4) | 0.53 |
| Baseline C3 (sd) | 59.6 (21) | 63.9 (27.1) | 0.27 |
| Baseline C4 (sd) | 9.66 (6.31) | 10.2 (7.12) | 0.64 |
| BIOPSY | |||
| CLASS (%) | 0.71 | ||
| • II | 2 (2.5) | 1 (1.2) | |
| • III | 14 (17.5) | 9 (11.2) | |
| • IV | 45 (56.2) | 48 (60) | |
| • V | 4 (5) | 2 (2.5) | |
| • MIXED | 15 (18.7) | 20 (25) | |
| AI mean (sd) | 7.05 (4.58) | 7.41 (3.57) | 0.42 |
| CI mean (sd) | 1.91 (1.97) | 1.93 (1.78) | 0.78 |
| INDUCTION TREATMENT | |||
| CYC (%) | 19 (23.7) | 13 (16.25) | 0.24 |
| AZA (%) | 0 (0) | 1 (1.25) | |
| MMF (%) | 55 (68.7) | 53 (66.2) | |
| CNI (%) | 2 (2.5) | 1 (1.25) | |
| RTX (%) | 4 (5) | 11 (13.7) | |
| MMF+CNI (%) | 0 (0) | 1 (1.25) | |
| HCQ (%) | 42 (56) | 60 (75) | |
AI, activity index; AZA, azathioprine; CI, chronicity index; CNI, calcineurin inhibitors; CYC, cyclophosphamide; HCQ, hydroxychloroquine; MMF, mycophenolate; MTX, methotrexate; RTX, rituximab
Proportion of patients achieving early complete renal response (CRR) (left) and prednisolone equivalent dose at complete renal response (right) in the control and belimumab cohorts after propensity score matching.
REFERENCES: [1] Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020; 79: 713–723.
Acknowledgements: NIL.
Disclosure of Interests: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (