

Background: Pozdeutinurad (AR882) is a novel and selective URAT1 inhibitor currently in Phase 3 clinical stage development for the treatment of gout and tophaceous gout. In a phase 2 trial in patients with chronic gouty arthritis, the 6-month core study was followed by two 6-month extension phases, resulting in a study duration of 18 months. The trial evaluated the treatment effects of pozdeutinurad alone or in combination with allopurinol on reduction in serum urate (sUA) and reduction in clinically visible subcutaneous tophi as measured by digital caliper.
Objectives: To evaluate the sustainability of treatment effect of pozdeutinurad in patients with chronic gouty arthritis and clinically visible tophi.
Methods: The phase 2, randomized, open-label, global trial, recruited 42 patients with at least one subcutaneous tophus. The patients were initially randomized equally into three treatment groups to receive pozdeutinurad 75 mg once daily (QD), pozdeutinurad 50 mg + allopurinol QD, or allopurinol up to 300 mg QD in the first 6 months (core phase). Those in the allopurinol alone group who opted in the 1st extension had pozdeutinurad 75 mg added to their current regimen. Those in the pozdeutinurad 50 mg + allopurinol group who opted in the 2nd extension were switched to pozdeutinurad 75 mg + allopurinol. Tophi measurements using digital calipers were completed every 4 weeks for the first 6 months followed by every 3 months in the extension phases. Efficacy endpoints included change in sUA from baseline and complete resolution of target tophus.
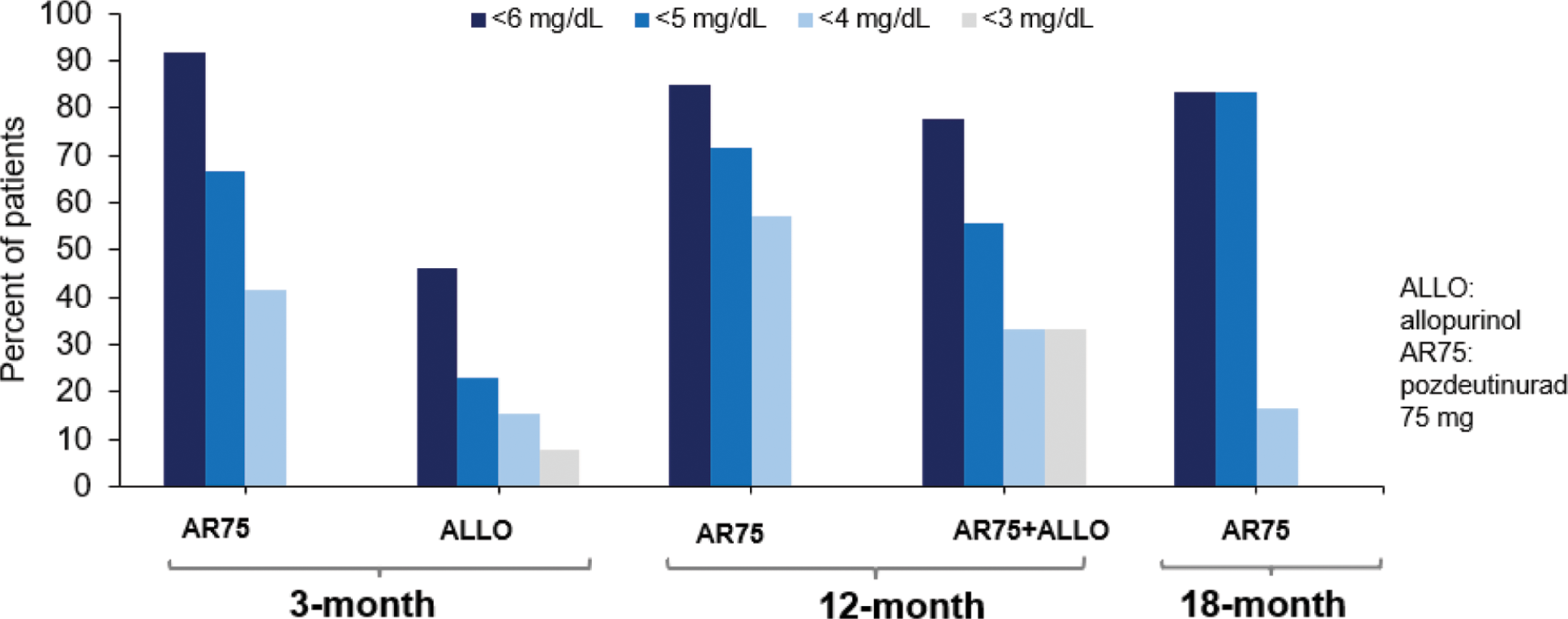
Results: Pozdeutinurad 75 mg alone showed sustained sUA lowering effect from Month 3 through Month 18. Median sUA levels were reduced from baseline 9.2 mg/dL to 4.6, 3.9 and 4.3 mg/dL, respectively at Months 3, 12 and 18. The corresponding response rates to achieve sUA < 6, 5, and 4 mg/dL were 92%, 67% and 42% at Month 3, 85%, 72% and 57% at Month 12 and 83%, 83% and 17% at Month 18, respectively (Figure 1). Allopurinol alone showed 46%, 23% and 15% of patients achieved sUA < 6, 5, and 4 mg/dL, respectively at Month 3. In the extension phases, adding pozdeutinurad 75 mg to allopurinol reduced sUA (5.9 mg/dL at Month 6) to 4.3 mg/dL (78%, 56% and 33% < 6, 5, 4 mg/dL, respectively at Month 18) with similar effect to pozdeutinurad alone. Both pozdeutinurad 75 mg alone or in combination with allopurinol achieved >40% complete resolution of target tophi or non-target tophi (Table 1).
Conclusion: The long-term treatment of pozdeutinurad in patients with tophaceous gout demonstrated sustained and efficacious sUA lowering and continued tophus resolution from the initial 6 months of treatment through 18 months of treatment. Pozdeutinurad alone or combination may provide an effective treatment option for patients with chronic gout, including those with clinically visible tophi that are difficult to treat.
Pozdeutinurad maintained high response rates to treatment up to 18 months of treatment
Pozdeutinurad showed high response rates of complete resolution in both target and non-target tophi up to 18 months
| Endpoint | Phase | Allopurinol
| Pozdeutinurad 75 mg
| Pozdeutinurad 75 mg +Allopurinol*
|
|---|---|---|---|---|
| Target tophi | 6-mo core | 1/13 (7.7%) | 4/12 (33.3%) | - |
| 6-mo 1st ext | - | 4/10 (40.0%) | 3/12 (25.0%) | |
| 6-mo 2nd ext | - | 3/6 (50.0%) | 4/9 (44.4%) | |
| Non-target tophi | 6-mo core | 0/9 (0) | 2/9 (22.2%) | - |
| 6-mo 1st ext | - | 3/7 (42.9%) | 2/8 (25.0%) | |
| 6-mo 2nd ext | - | 2/4 (50.0%) | 2/5 (40.0%) |
*subjects were originally assigned allopurinol treatment at baseline with pozdeutinurad 75 mg added after 6 months; ext: extension; mo: month
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Robert Keenan Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Elizabeth Polvent Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Sarah Morris Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Pamela Mundell Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Wen Wei Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Zancong Shen Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Vijay Hingorani Arthrosi Therapeutics Inc, Shunqi Yan Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc, Li-Tain Yeh Arthrosi Therapeutics Inc, Arthrosi Therapeutics Inc.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (