

Background: Although the majority of healthcare professionals are convinced of the quality, safety, and favorable economic impact of biosimilars, their routine use in practice is not yet widespread (Jarrion et al.2022; EMA 2017). FK-toci (MSB11456) is the first tocilizumab biosimilar approved both in IV and sub-cutaneous routes. Its biosimilarity to the originator has been demonstrated in an extensive non-clinical and clinical program which included pharmacokinetic, efficacy, safety and immunogenic profile in patients with moderate to severe rheumatoid arthritis (RA) (APTURA I study, Sienkiewicz et al. 2023).
Objectives: The overall aim of RUBY, a multinational, prospective non-interventional study is to evaluate the real world use of FK-toci (either in initiation or switch) in RA patients over 12 months, assess persistence rate at 6 months after treatment initiation, and identify factors associated with treatment persistence for at least 12 months in order to develop a predictive model.
Methods: Data collected include patient characteristics, reasons for treatment switch, and clinical status at each RA adult patients were included, those participating or expected to participate in any interventional clinical trial during their treatment with FK-toci were excluded. The primary endpoint is the persistence on FK-toci 6 months after treatment start. Data collected include basic clinical characteristics, chronic inflammatory disease history, and current clinical status including disease activity score (DAS28-CRP), Patient Global Assessment, Physician Global Assessment and adverse events at each follow-up visit up to 12 months after treatment start. Data are presented using descriptive statistics and a logistic regression model will be employed to identify potential predictive factors of persistence at 12 months. We present here the baseline patients characteristics, and the 6 months intermediate results.
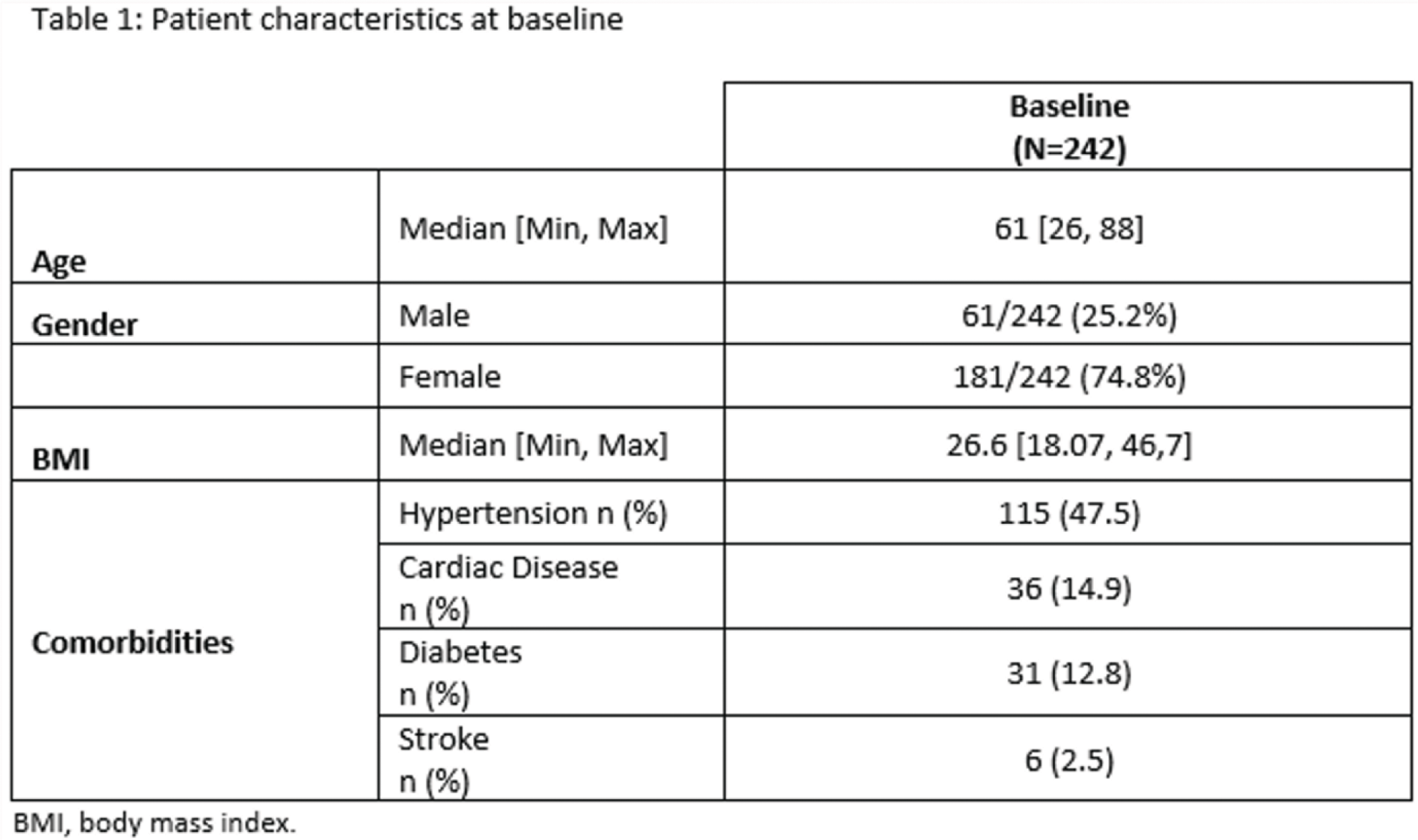
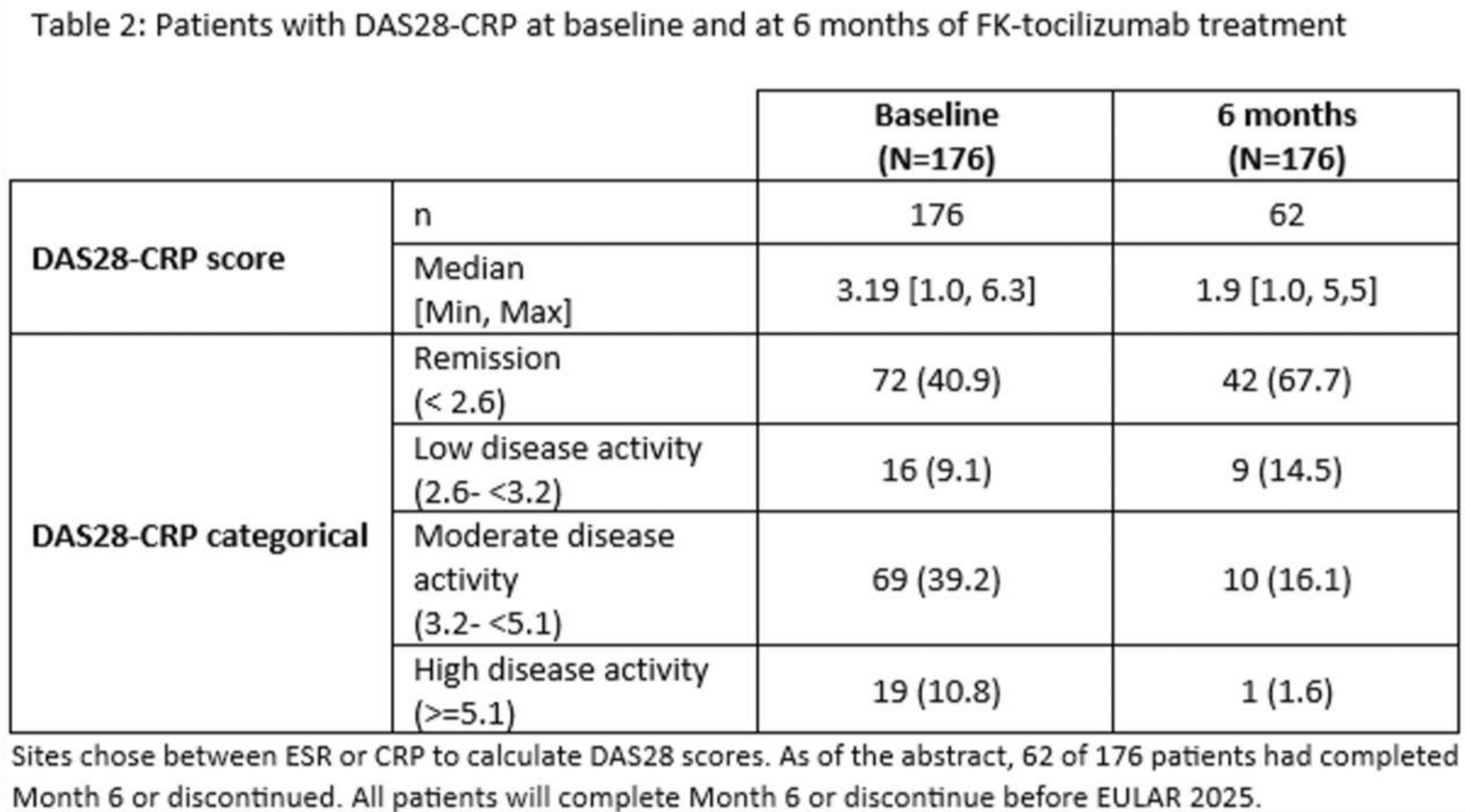
Results: A total of 242 RA patients in Germany (219), UK (18) and Spain (5) completed the 6 month follow-up visit. At study start, the median duration of RA disease was 10.6 years. 78.5% patients were naïve to tocilizumab while 21.5% switched from the originator. Patient characteristics at baseline, are summarized in Table 1. The most frequent comorbidity was hypertension (47.5%) and most frequent extra-articular manifestations were rheumatoid skin nodules (8.7%), Raynaud’s phenomenon (2.1%) and pulmonary fibrosis (2.1%). FK-toci was administered mainly by subcutaneous route (76.4%) using often the autoinjector (50.8%). At the start of the treatment, FK-toci was administered as monotherapy in more than half of patients (55.8%). The persistence rate of FK-toci at 6 months was 81.5%. Permanent treatment discontinuations occurred primarily due to adverse events. The disease activity assessed by the DAS28-CRP score was improved at 6 months with higher rates of low-disease activity and remission (Table 2). This improvement is also reflected in the Global patient and Physician Assessment with median scores decreasing from 50 to 40 and from 30 to 10, respectively. Since the start of the study 92 patients (38%) experienced at least one adverse event (AE); and 46 (19%) had AE(s) possibly attributable to FK-toci. AE(s) possibly attributable to FK-toci lead to permanent discontinuation for 13 (5.4%) patients.
Conclusion: RUBY is a non-interventional study designed to evaluate the real-world use of the first tocilizumab biosimilar in RA patients in Europe. Intermediate results show high persistency at 6-months with improved response rates. Identification of factors influencing treatment persistence at 12 months in either switched or newly initiated patients will support doctors in the use of tocilizumab biosimilars in the routine management of RA patients.
REFERENCES: [1] Jarrion Q, Azzouz B, Robinson J, Jolly D, Vallet C, Trenque T. Penetration rate of anti-TNF biosimilars and savings at 5 years after their introduction in French hospitals. Therapie. 2022;77(4):467–75. doi:10.1016/j.therap.2021.10.012.
[2] Zubrzycka Sienkiewicz A. et al. Comparison of the efficacy and safety of a proposed: biosimilar MSB11456 with tocilizumab reference product in moderate to severe rheumatoid arthritis: results of a randomized double-blind study. (abstract) Arthritis Rheumatol. 2023; 75 (suppl 9).
Acknowledgements: NIL.
Disclosure of Interests: Graziella Pourcel Fresenius kabi, Eugen Feist: None declared, Ioana Andreica: None declared, Ernest Wong: None declared, María América López Lasanta: None declared, Fabrizio Dolfi: None declared, Joëlle Monnet: None declared, Peter Baker: None declared, Maria ROMANOVA: None declared.
© The Authors 2025. This abstract is an open access article published in Annals of Rheumatic Diseases under the CC BY-NC-ND license (